Answered step by step
Verified Expert Solution
Question
1 Approved Answer
reaction of chemical engineering 1. The reactions 1 and 2 should be 2AB for rection 1 , and A2C for reaction 2 . 2. Instead
reaction of chemical engineering 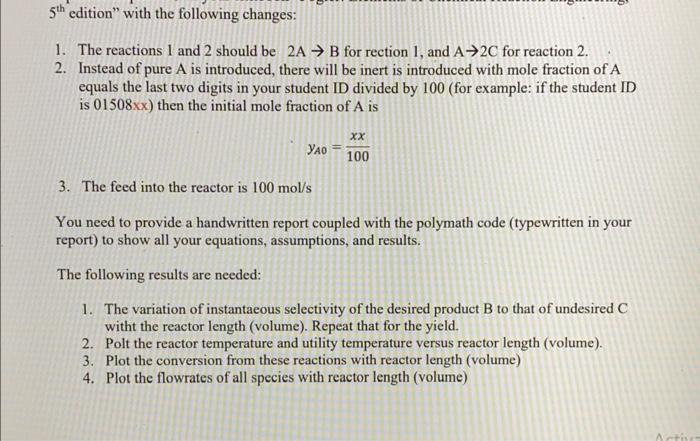
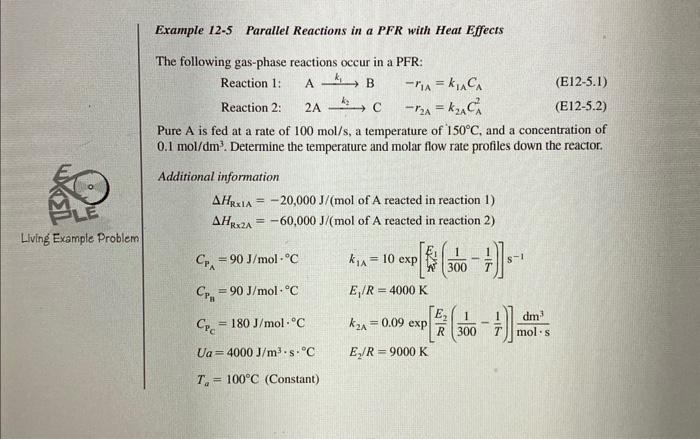
1. The reactions 1 and 2 should be 2AB for rection 1 , and A2C for reaction 2 . 2. Instead of pure A is introduced, there will be inert is introduced with mole fraction of A equals the last two digits in your student ID divided by 100 (for example: if the student ID is 01508x ) then the initial mole fraction of A is yA0=100xx 3. The feed into the reactor is 100mol/s You need to provide a handwritten report coupled with the polymath code (typewritten in your report) to show all your equations, assumptions, and results. The following results are needed: 1. The variation of instantaeous selectivity of the desired product B to that of undesired C witht the reactor length (volume). Repeat that for the yield. 2. Polt the reactor temperature and utility temperature versus reactor length (volume). 3. Plot the conversion from these reactions with reactor length (volume) 4. Plot the flowrates of all species with reactor length (volume) Example 12-5 Parallel Reactions in a PFR with Heat Effects The following gas-phase reactions occur in a PFR: Reaction 1: Ak1Br1A=k1ACA (E125.1) Reaction 2: 2Ak2Cr2A=k2ACA2 (E12-5.2) Pure A is fed at a rate of 100mol/s, a temperature of 150C, and a concentration of 0.1mol/dm3. Determine the temperature and molar flow rate profiles down the reactor. Additional information HR1A=20,000J/(molofAreactedinreaction1)HR2A=60,000J/(molofAreactedinreaction2)CPA=90J/molCk1A=10exp[kkE1(3001T1)]s1CPn=90J/molCE1/R=4000KCPC=180J/molCk2A=0.09exp[RE2(3001T1)]molsdm3Ua=4000J/m3sCE2/R=9000KTa=100C(Constant) 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


