Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Read Section 1 1 . 5 . You can click on the Review link to access the section in your eText. The vapor pressure of
Read Section You can click on the Review link to access the section in your eText.
The vapor pressure of at is torr.
Part A
If of is enclosed in a container, calculate the number of moles of in the gas phase. Express your answer using two significant figures.
Previous Answers
Part B
Given your answer to Part A will any liquid be present?
yes
no
Previous Answers
Correct
There are mol of liquid present initially and mol of gas present at equilibrium. So yes, there will be liquid present because not all of the liquid went into the gas phase.
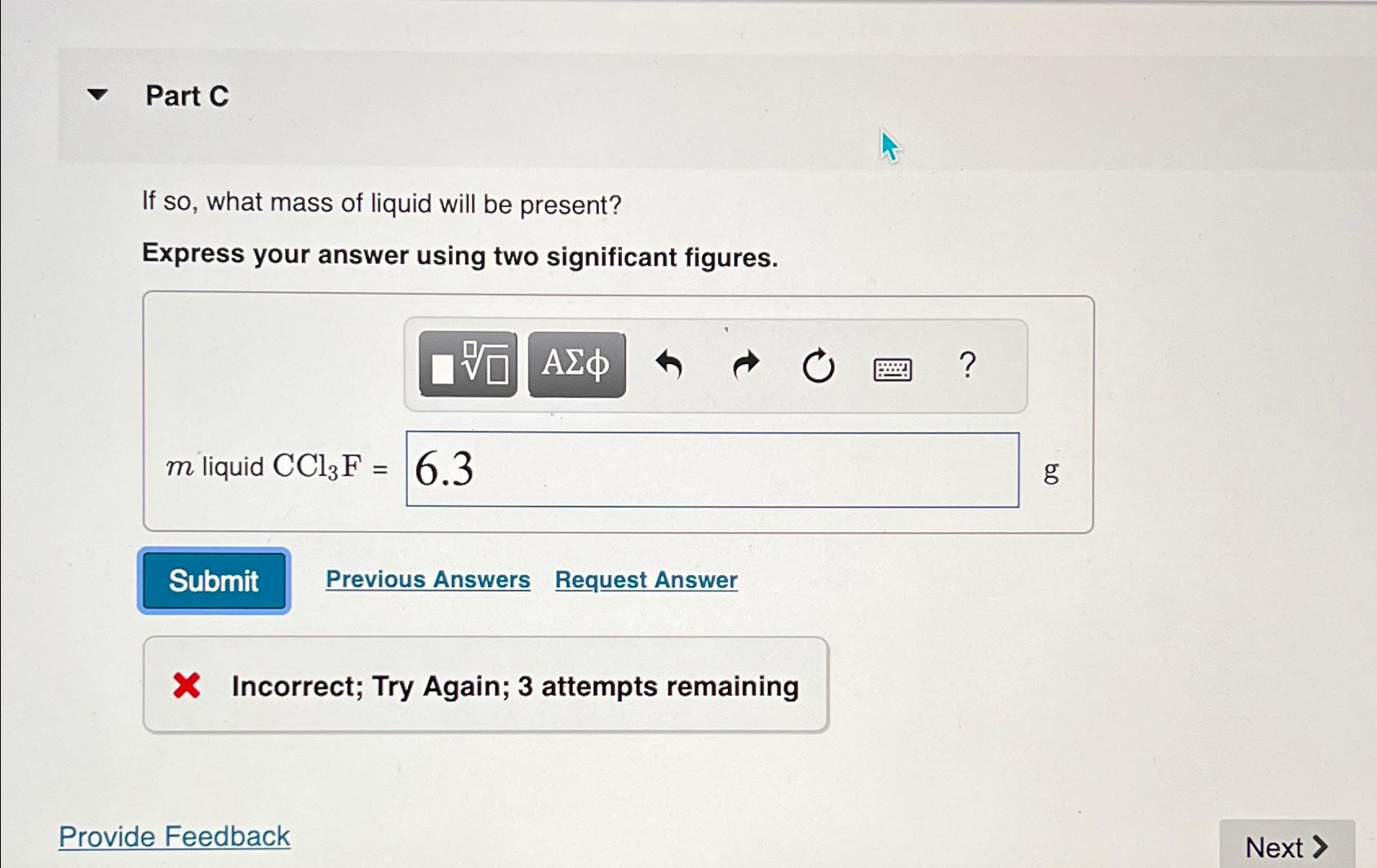
Part C
If so what mass of liquid will be present?
Express your answer using two significant figures.
liquid
Previous Answers
Request Answer
Incorrect; Try Again; attempts remaining
Provide Feedback
Answer all three please
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


