Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Really lost could use some guidance 2. Consider the novel nanostructured catalyst surface shown in the figure below. The catalyst suppoet consists of an ordered
Really lost could use some guidance 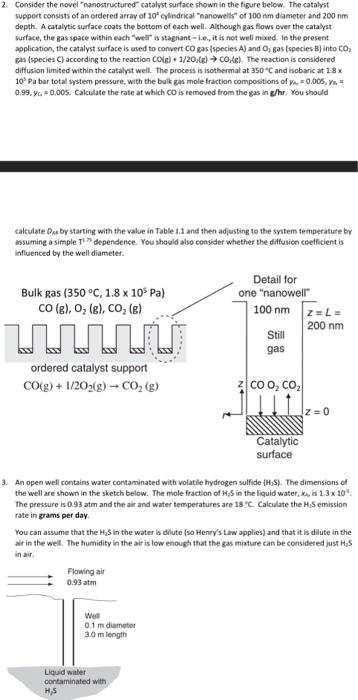
2. Consider the novel "nanostructured" catalyst surface shown in the figure below. The catalyst suppoet consists of an ordered array of 102 cylindrical "nanowelk" of 100 nm diameter and 200 nm depth. A catalytic surface coats the bottom of each well. Although gas flows over the catalyst surface, the gas space wishin each "wedr" is stagnant - i.e. it is not well mixed. In the present application, the catalyst surface is used to convert CO gas (species A ) and O2 gas (species B) into CO2 gas (species C ) according to the reaction CO(g)+1/2O2(g)CO/B). The reaction is considered diffusion limited within the catalyst well. The process is isothermal at 350C and lsabaric at 18x 105 Pa bar total system pressure, with the bulk gas mole fraction compositions of y4=0.005,= 0.99,yc=0.005. Calculate the rate at which CO is removed from the gas in g/hr. You should calculate Dss by starting with the value in Table 1.1 and then adjusting to the system temperature by assuming a simple Tt dependence. You should also consider whether the diffusion coefficient is influenced by the well diameter. 3. An open well contains water contaminated with volatile hydrogen sulfide {H4S}. The dimensions of the well are shown in the sketch below. The mole fraction of 425 in the liquid water, x.,61.3x1104. The pressure is 0.93 atm and the alr and water temperatures are 18C. Calculate the H1S emission rate in grams per day. You can assume that the H2S in the water ls dilute (so Henry's Lw applies) and that it is dillute in the air in the well. The humidity in the air is low enough that the gas miature can be considered just H. 5 in air 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


