Question
A sample of an unknown gas requires 45 seconds to effuse from a container. Under identical conditions, a sample of bromine gas effuses in

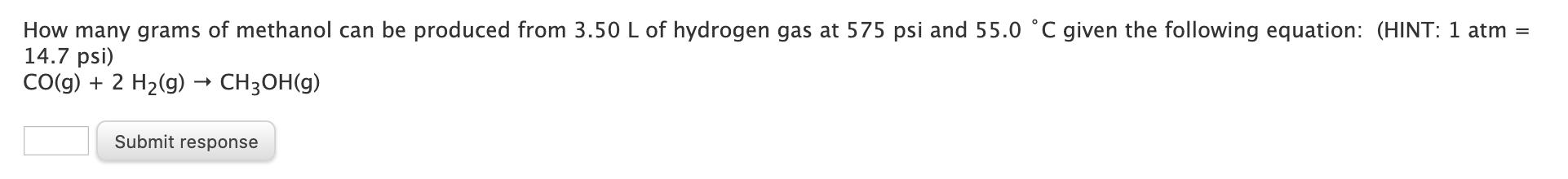
A sample of an unknown gas requires 45 seconds to effuse from a container. Under identical conditions, a sample of bromine gas effuses in 90 seconds. You know the unknown gas must have come from a gas tank in your lab. What is the identity of the unknown gas? A. Helium B. Neon C. Argon D. Oxygen E. Chlorine F. lodine G. Krypton H. Xenon How many grams of methanol can be produced from 3.50 L of hydrogen gas at 575 psi and 55.0 C given the following equation: (HINT: 1 atm = 14.7 psi) CO(g) + 2 H(g) CH3OH(g) Submit response
Step by Step Solution
3.38 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
Find the molar mass of unknown gas and ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Managerial economics
Authors: william f. samuelson stephen g. marks
7th edition
9781118214183, 1118041585, 1118214188, 978-1118041581
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



