Answered step by step
Verified Expert Solution
Question
1 Approved Answer
REDUCTION OF BENZIL USING SODIUM BOROHYDRIDE: (95% ethanol was used in this process) Based on the masses and data provided in the picture calculate and
REDUCTION OF BENZIL USING SODIUM BOROHYDRIDE: (95% ethanol was used in this process)
-the limiting reagent
-the theoretical yield
-the percent yield
*please be sure to explain where the numbers are coming from- as I am a little confused as to how to find the limiting reagent and theoretical yield*
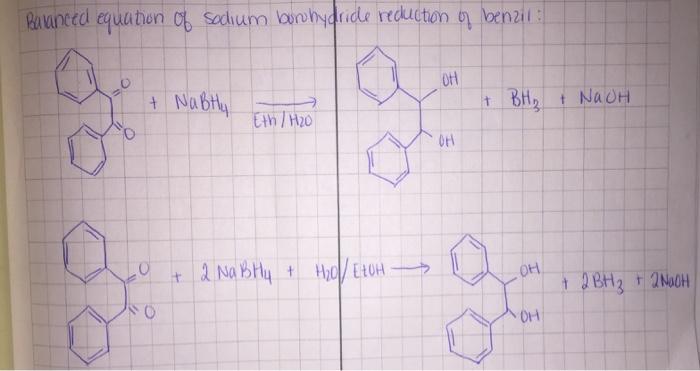
Additional information such as chemical equation is provided in subsequent picture.

masses 125-ml Erlenmeyer flask = 83,659 watch glass - 63.821g Benzil = 0.9749 NaBH4 = 0.2129 mass of product + watch glass after drying = 64.485g melting point of product = 135-139C. melting point of product + benzuin (mixecl mp) = 133-135C melting point of product + meso-hydrobenzoin = 136-138 C major product = meso-hydro benzoin calculate: - limiting reagent theoretical yield percent yield
Step by Step Solution
★★★★★
3.47 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
Benzil 0974g Moles of Benzil 21022 000463 moles Limiting reagent is benzil since NaBHy is ta...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


