Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Refer to image attached: Og is the noble gas after Rn. To go from [Rn] to [Og], you must fill four subshells (s,p,d, and f)
Refer to image attached:
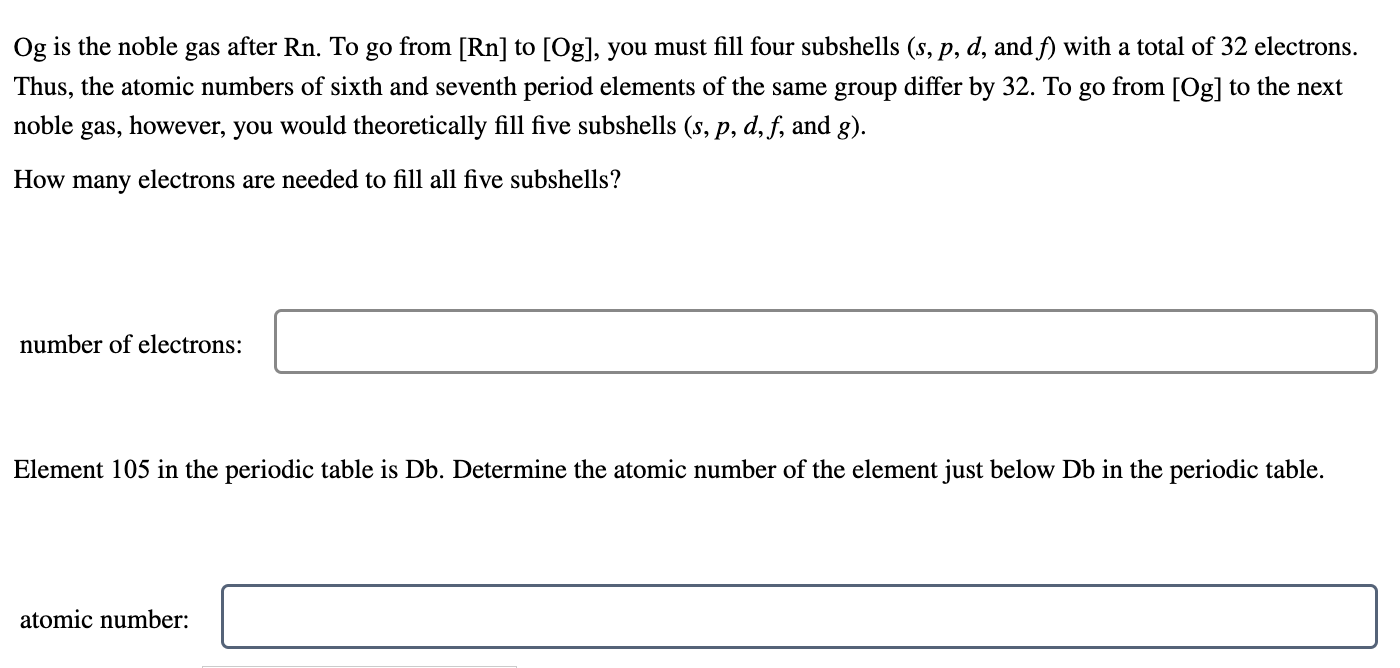
Og is the noble gas after Rn. To go from [Rn] to [Og], you must fill four subshells (s,p,d, and f) with a total of 32 electrons. Thus, the atomic numbers of sixth and seventh period elements of the same group differ by 32 . To go from [Og] to the next noble gas, however, you would theoretically fill five subshells (s,p,d,f, and g ). How many electrons are needed to fill all five subshells? number of electrons: Element 105 in the periodic table is Db. Determine the atomic number of the element just below Db in the periodic table. atomic number
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


