Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Look at the given synthesis and the provided reagents. Fill in the blanks with the single letter codeof the appropriate reagent, or the appropriate

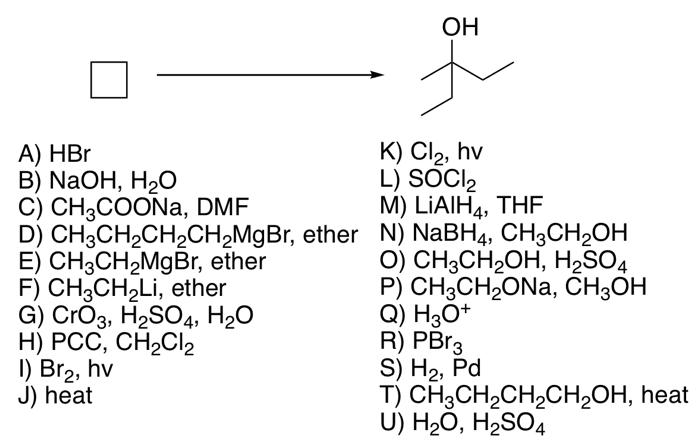

Look at the given synthesis and the provided reagents. Fill in the blanks with the single letter codeof the appropriate reagent, or the appropriate descriptive vocabulary word/phrase for non-reagents to complete the synthesis and description. spelling. carefully! OH A) HBr B) NaOH, HO C) CH3COONa, DMF K) Cl2, hv L) SOCI M) LiAlH4, THF D) CH3CH2CH2CH2MgBr, ether N) NaBH4, CH3CH2OH E) CH3CH2MgBr, ether F) CH3CH2Li, ether G) CrO3, H2SO4, HO H) PCC, CH2Cl2 I) Br2, hv J) heat O) CH3CH2OH, H2SO4 P) CH3CH2ON, CH3OH Q) H3O+ R) PB3 S) H2, Pd T) CH3CH2CH2CH2OH, heat U) H2O, H2SO4 -First we add reagent S to form the molecule butane (IUPAC name). -Then reagent / to form a bromo functional group in a free radical bromination reaction. -This can be converted to a functional group hydroxyl by first adding reagent C in a SN2 mechanism then following up with reagent U to give the desired group via a Ester Hydrolysis reaction. -A(n) oxidation (oxidation, reduction, substitution, hydrolysis, other) with reagent H will give the functional group carbonyl, which can be reacted first with E followed by reagent Q workup to give the desired product.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Solutions Step 1 Oxidizing agent ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


