Answered step by step
Verified Expert Solution
Question
1 Approved Answer
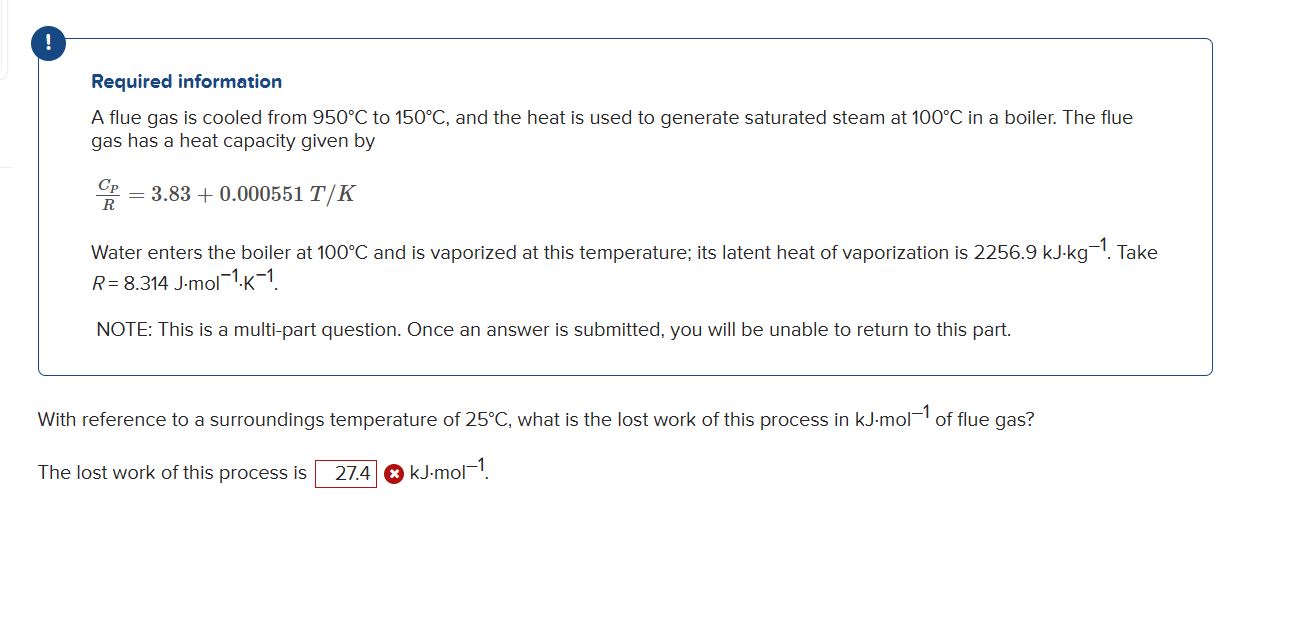
! Required information A flue gas is cooled from 9 5 0 C to 1 5 0 C , and the heat is used to
Required information
A flue gas is cooled from to and the heat is used to generate saturated steam at in a boiler. The flue
gas has a heat capacity given by
Water enters the boiler at and is vaporized at this temperature; its latent heat of vaporization is Take
NOTE: This is a multipart question. Once an answer is submitted, you will be unable to return to this part.
With reference to a surroundings temperature of what is the lost work of this process in of flue gas?
The lost work of this process is
A flue gas is cooled from deg C to deg C and the heat is used to generate saturated steam at deg C in a boiler. The flue gas has a heat capacity given by
CPR TK
Water enters the boiler at deg C and is vaporized at this temperature; its latent heat of vaporization is kJkg Take R JmolK
NOTE: This is a multipart question. Once an answer is submitted, you will be unable to return to this part.
With reference to a surroundings temperature of deg C what is the lost work of this process in kJmol of flue gas?
The lost work of this process is
kJmol
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


