Question
reversible heating and work: Tds = we have from the 1st and 2nd Laws of Thermodynamics for de + pd (1) = dh -
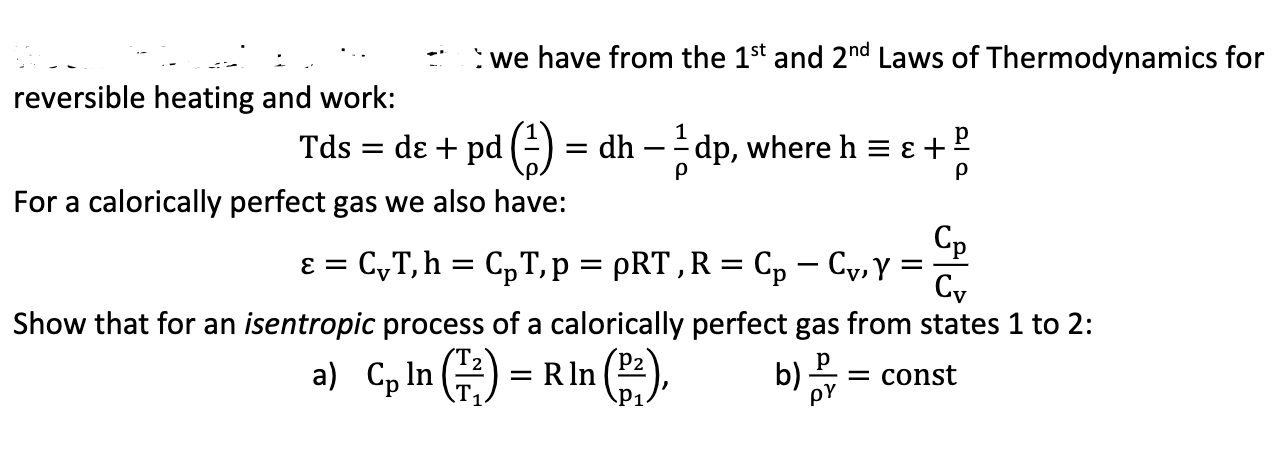
reversible heating and work: Tds = we have from the 1st and 2nd Laws of Thermodynamics for de + pd (1) = dh - 1 dp, where h = + For a calorically perfect gas we also have: = CT, h = CpT, p = pRT, R = Cp Cv, Y = Cv Show that for an isentropic process of a calorically perfect gas from states 1 to 2: a) Cp In (2) = R In P1 b) = const
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals Of Aerodynamics
Authors: John Anderson
6th Edition
1259129918, 978-1259129919
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



