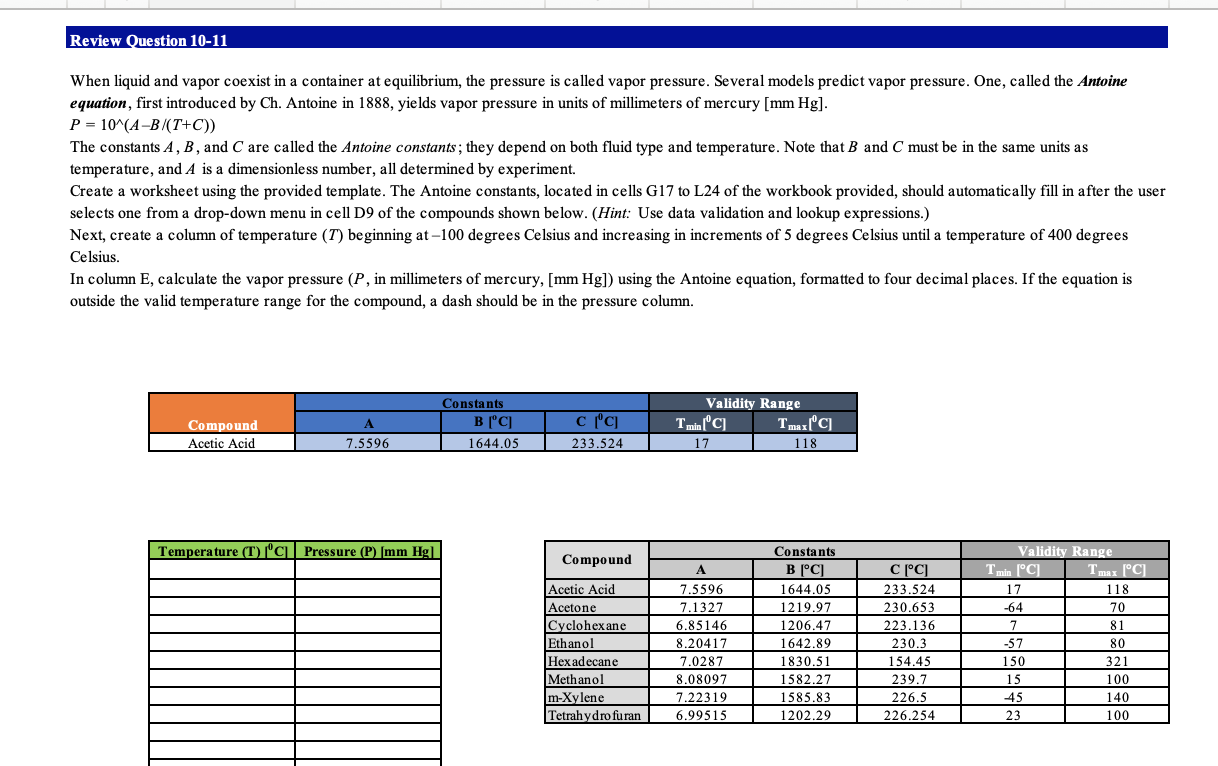

Review Question 10-11 When liquid and vapor coexist in a container at equilibrium, the pressure is called vapor pressure. Several models predict vapor pressure. One, called the Antoine equation, first introduced by Ch. Antoine in 1888, yields vapor pressure in units of millimeters of mercury (mm Hg). P = 10^(A-B/T+C)) The constants A, B, and C are called the Antoine constants; they depend on both fluid type and temperature. Note that B and C must be in the same units as temperature, and A is a dimensionless number, all determined by experiment. Create a worksheet using the provided template. The Antoine constants, located in cells G17 to L24 of the workbook provided, should automatically fill in after the user selects one from a drop-down menu in cell D9 of the compounds shown below. (Hint: Use data validation and lookup expressions.) Next, create a column of temperature (T) beginning at -100 degrees Celsius and increasing in increments of 5 degrees Celsius until a temperature of 400 degrees Celsius. In column E, calculate the vapor pressure (P, in millimeters of mercury, [mm Hg]) using the Antoine equation, formatted to four decimal places. If the equation is outside the valid temperature range for the compound, a dash should be in the pressure column. Compound Acetic Acid Constants Validity Range A B | CITminCTIC 7.559611644.05 1 233.524 17 1118 1 Temperature (T) /'c Pressure (P) (mm Hg1 Compound 70 Validity Range T min [C] Tmax [C] 17 118 -64 81 -57 80 Acetic Acid Acetone Cyclohexane Ethanol Hexadecane Methanol m-Xylene Tetrahydrofuran Constants (C) 1644.05 1219.97 1206.47 1642.89 1830.51 1582.27 1585.83 1202.291 7.5596 7.1327 6.85146 8.20417 7.0287 8.08097 7.22319 6.99515 CC 233.524 230.653 223.136 230.3 154.45 239.7 226.5 226.254 150 321 15 45 23 100 140 100 1 Instructions 1 Start Excel. In cell D9, use data validation and absolute addressing to create a selection of the compounds from the list given in cells G17 - G24. Then select any compound from the drop-down list. Note: Keep the default settings for the Input Message tab and for the Error Alert tab. (2 points) In cells E9, F9, and G9, by using relative addressing, show the constants A, B and C, respectively, corresponding to the selected compound. Note: Use Excel function VLOOKUP. Use cells G17-L24 for the table array argument. Use the FALSE parameter for the last argument. (3 points) In cells H9 and 19, by using relative addressing, show the minimum and maximum temperature, respectively, corresponding to the valid temperature range for the selected compound. Note: Use Excel function VLOOKUP. Use cells G17-L24 for the table array argument. Use the FALSE parameter for the last argument. (2 points) 5 In cell D16, enter the starting value for the temperature. (1 point) In cell D17, use relative addressing to the cell directly above to obtain the next value with the increment of 5 degrees. Copy the formula from cell D17 down the column to cell D116. (1 point) In cell E16, by using relative and absolute addressing, calculate the vapor pressure by the formula: P = 10^(A-B/(T+C)). The cell must contain a dash if the equation is outside the valid temperature range. Use a compound conditional statement written in the following way: IF(AND(logical test #1, logical test #2), P, -), where 7 logical test #1 checks if the value in cell D16 is greater than the value in cell H9, logical test #2 checks if the value in cell D16 is less than the value in cell 19, P is the formula for the vapor pressure. Note: for logical test #1 and logical test #2 enter the expressions describing the relationships between the cells given above. Equivalent forms will not be graded. Copy the formula from cell E16 down the column to cell E116. (4 points) 8 Save the workbook. Close the workbook and then exit Excel. Submit the workbook as directed. Review Question 10-11 When liquid and vapor coexist in a container at equilibrium, the pressure is called vapor pressure. Several models predict vapor pressure. One, called the Antoine equation, first introduced by Ch. Antoine in 1888, yields vapor pressure in units of millimeters of mercury (mm Hg). P = 10^(A-B/T+C)) The constants A, B, and C are called the Antoine constants; they depend on both fluid type and temperature. Note that B and C must be in the same units as temperature, and A is a dimensionless number, all determined by experiment. Create a worksheet using the provided template. The Antoine constants, located in cells G17 to L24 of the workbook provided, should automatically fill in after the user selects one from a drop-down menu in cell D9 of the compounds shown below. (Hint: Use data validation and lookup expressions.) Next, create a column of temperature (T) beginning at -100 degrees Celsius and increasing in increments of 5 degrees Celsius until a temperature of 400 degrees Celsius. In column E, calculate the vapor pressure (P, in millimeters of mercury, [mm Hg]) using the Antoine equation, formatted to four decimal places. If the equation is outside the valid temperature range for the compound, a dash should be in the pressure column. Compound Acetic Acid Constants Validity Range A B | CITminCTIC 7.559611644.05 1 233.524 17 1118 1 Temperature (T) /'c Pressure (P) (mm Hg1 Compound 70 Validity Range T min [C] Tmax [C] 17 118 -64 81 -57 80 Acetic Acid Acetone Cyclohexane Ethanol Hexadecane Methanol m-Xylene Tetrahydrofuran Constants (C) 1644.05 1219.97 1206.47 1642.89 1830.51 1582.27 1585.83 1202.291 7.5596 7.1327 6.85146 8.20417 7.0287 8.08097 7.22319 6.99515 CC 233.524 230.653 223.136 230.3 154.45 239.7 226.5 226.254 150 321 15 45 23 100 140 100 1 Instructions 1 Start Excel. In cell D9, use data validation and absolute addressing to create a selection of the compounds from the list given in cells G17 - G24. Then select any compound from the drop-down list. Note: Keep the default settings for the Input Message tab and for the Error Alert tab. (2 points) In cells E9, F9, and G9, by using relative addressing, show the constants A, B and C, respectively, corresponding to the selected compound. Note: Use Excel function VLOOKUP. Use cells G17-L24 for the table array argument. Use the FALSE parameter for the last argument. (3 points) In cells H9 and 19, by using relative addressing, show the minimum and maximum temperature, respectively, corresponding to the valid temperature range for the selected compound. Note: Use Excel function VLOOKUP. Use cells G17-L24 for the table array argument. Use the FALSE parameter for the last argument. (2 points) 5 In cell D16, enter the starting value for the temperature. (1 point) In cell D17, use relative addressing to the cell directly above to obtain the next value with the increment of 5 degrees. Copy the formula from cell D17 down the column to cell D116. (1 point) In cell E16, by using relative and absolute addressing, calculate the vapor pressure by the formula: P = 10^(A-B/(T+C)). The cell must contain a dash if the equation is outside the valid temperature range. Use a compound conditional statement written in the following way: IF(AND(logical test #1, logical test #2), P, -), where 7 logical test #1 checks if the value in cell D16 is greater than the value in cell H9, logical test #2 checks if the value in cell D16 is less than the value in cell 19, P is the formula for the vapor pressure. Note: for logical test #1 and logical test #2 enter the expressions describing the relationships between the cells given above. Equivalent forms will not be graded. Copy the formula from cell E16 down the column to cell E116. (4 points) 8 Save the workbook. Close the workbook and then exit Excel. Submit the workbook as directed








