Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Review The goal in this problem is to estimate the strength of an interatomic bond in silver. We will model a thin, silver wire
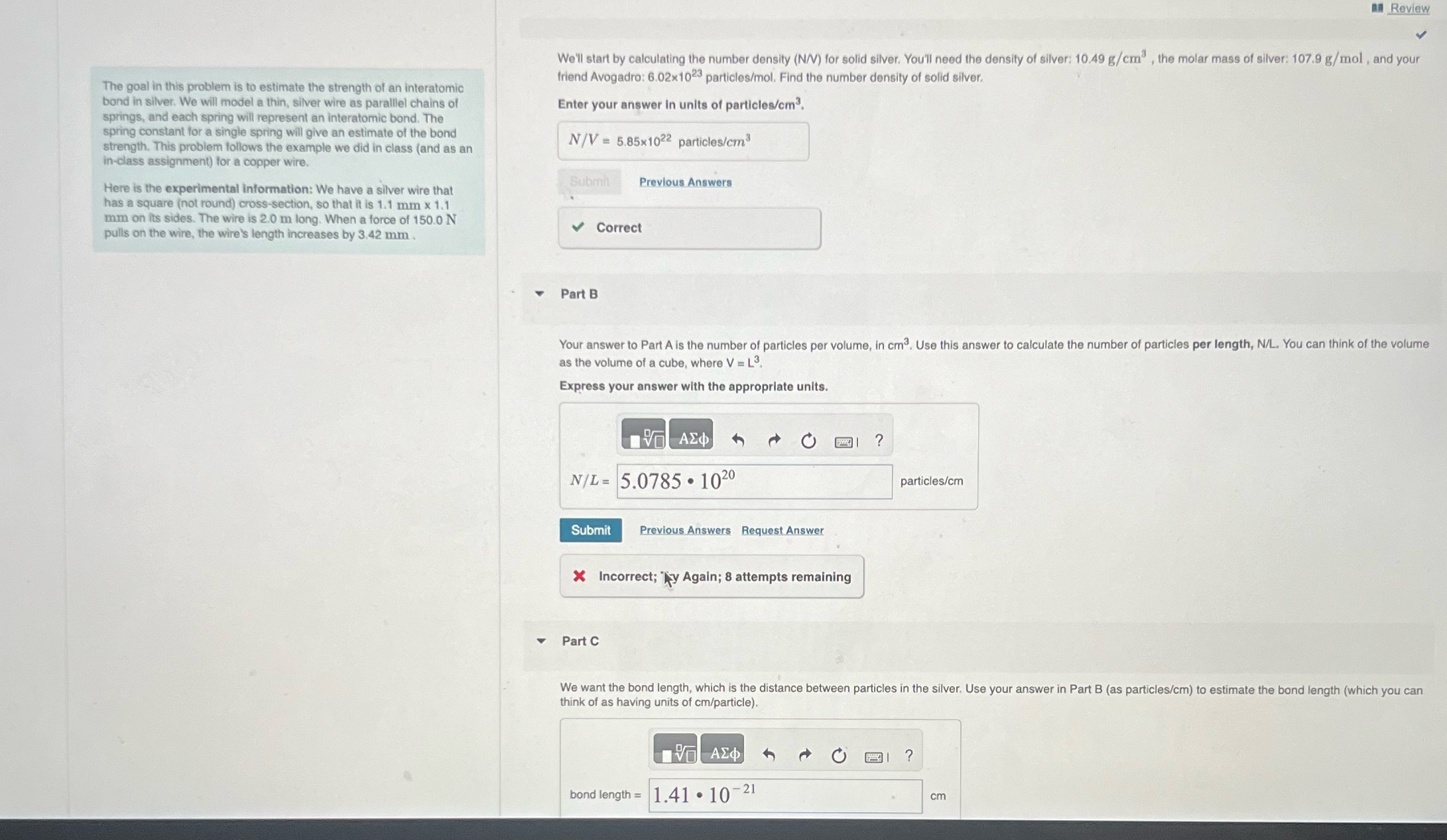
Review The goal in this problem is to estimate the strength of an interatomic bond in silver. We will model a thin, silver wire as parallel chains of springs, and each spring will represent an interatomic bond. The spring constant for a single spring will give an estimate of the bond strength. This problem follows the example we did in class (and as an in-class assignment) for a copper wire. Here is the experimental information: We have a silver wire that has a square (not round) cross-section, so that it is 1.1 mm x 1.1 mm on its sides. The wire is 2.0 m long. When a force of 150.0 N pulls on the wire, the wire's length increases by 3.42 mm. We'll start by calculating the number density (N/V) for solid silver. You'll need the density of silver: 10.49 g/cm, the molar mass of silver: 107.9 g/mol, and your friend Avogadro: 6.02x1023 particles/mol. Find the number density of solid silver. Enter your answer in units of particles/cm. N/V=5.85x1022 particles/cm Submit Previous Answers Correct 4 Part B Your answer to Part A is the number of particles per volume, in cm. Use this answer to calculate the number of particles per length, N/L. You can think of the volume as the volume of a cube, where V = L. Express your answer with the appropriate units. N/L=5.0785 1020 Submit Previous Answers Request Answer Incorrect; y Again; 8 attempts remaining ? particles/cm Part C We want the bond length, which is the distance between particles in the silver. Use your answer in Part B (as particles/cm) to estimate the bond length (which you can think of as having units of cm/particle). bond length = 1.41 107 -21 ? cm
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


