Question
Ribose, a carbohydrate with the formula shown, forms a cyclic hemiacetal, which, in principle, could contain either a four-membered, five-membered, or six-membered ring. To
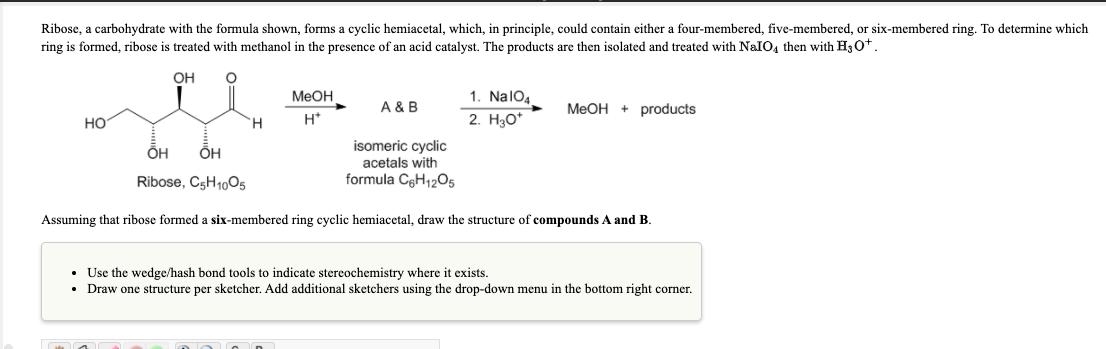
Ribose, a carbohydrate with the formula shown, forms a cyclic hemiacetal, which, in principle, could contain either a four-membered, five-membered, or six-membered ring. To determine which ring is formed, ribose is treated with methanol in the presence of an acid catalyst. The products are then isolated and treated with NaIO4 then with H30*. OH MeOH 1. NalO4 A & B MeOH + products H. H* 2. H30* H isomeric cyclic acetals with Ribose, C5H1005 formula CeH12O5 Assuming that ribose formed a six-membered ring cyclic hemiacetal, draw the structure of compounds A and B. Use the wedge/hash bond tools to indicate stereochemistry where it exists. Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner.
Step by Step Solution
3.42 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: Marc Loudon
5th edition
981519431, 978-0981519449, 098151944X, 978-0-98151943, 978-0981519432
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



