Answered step by step
Verified Expert Solution
Question
1 Approved Answer
roll no 156 so K=156/600 Q1. A RTD experiment was performed on a reactor to collect the concentration time data at ambient conditions. Reaction is
roll no 156 so K=156/600 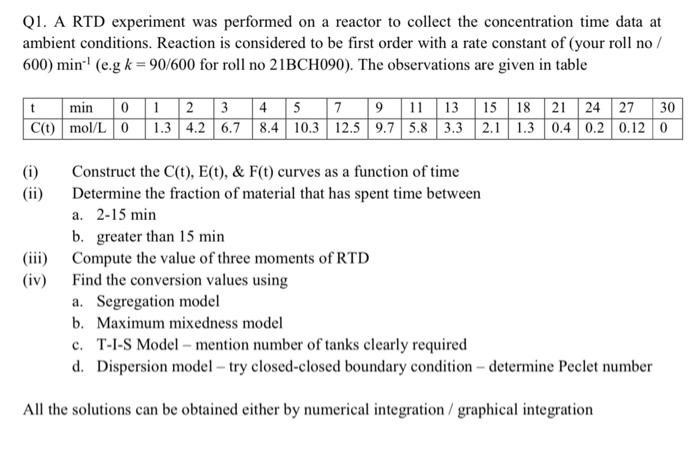
Q1. A RTD experiment was performed on a reactor to collect the concentration time data at ambient conditions. Reaction is considered to be first order with a rate constant of (your roll no / 600) min1 (e.g k=90/600 for roll no 21BCH090 ). The observations are given in table (i) Construct the C(t),E(t),&F(t) curves as a function of time (ii) Determine the fraction of material that has spent time between a. 215min b. greater than 15min (iii) Compute the value of three moments of RTD (iv) Find the conversion values using a. Segregation model b. Maximum mixedness model c. T-I-S Model - mention number of tanks clearly required d. Dispersion model - try closed-closed boundary condition - determine Peclet number 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


