Answered step by step
Verified Expert Solution
Question
1 Approved Answer
We will compare three different technologies for cars. Car A is a conventional gasoline-burning car, which converts about 15% of the fuel energy to
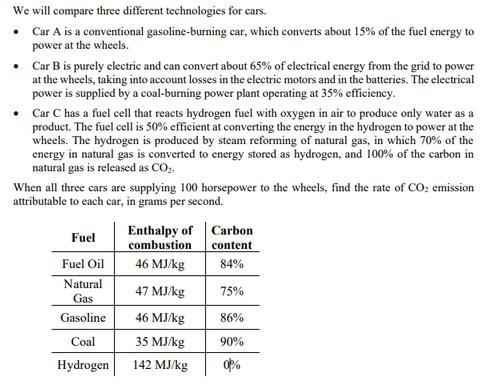
We will compare three different technologies for cars. Car A is a conventional gasoline-burning car, which converts about 15% of the fuel energy to power at the wheels. Car B is purely electric and can convert about 65% of electrical energy from the grid to power at the wheels, taking into account losses in the electric motors and in the batteries. The electrical power is supplied by a coal-burning power plant operating at 35% efficiency. Car C has a fuel cell that reacts hydrogen fuel with oxygen in air to produce only water as a product. The fuel cell is 50% efficient at converting the energy in the hydrogen to power at the wheels. The hydrogen is produced by steam reforming of natural gas, in which 70% of the energy in natural gas is converted to energy stored as hydrogen, and 100% of the carbon in natural gas is released as CO2. When all three cars are supplying 100 horsepower to the wheels, find the rate of CO2 emission attributable to each car, in grams per second. Enthalpy of Carbon Fuel combustion content Fuel Oil 46 MJ/kg 84% Natural 47 MJ/kg 75% Gas Gasoline 46 MJ/kg 86% Coal 35 MJ/kg 90% Hydrogen 142 MJ/kg 0%
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To find the rate of CO2 emission attributable to each car we need to calculate the amount of fuel consumed by each car per second and then calculate the corresponding CO2 emissions based on the carbon ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


