Answered step by step
Verified Expert Solution
Question
1 Approved Answer
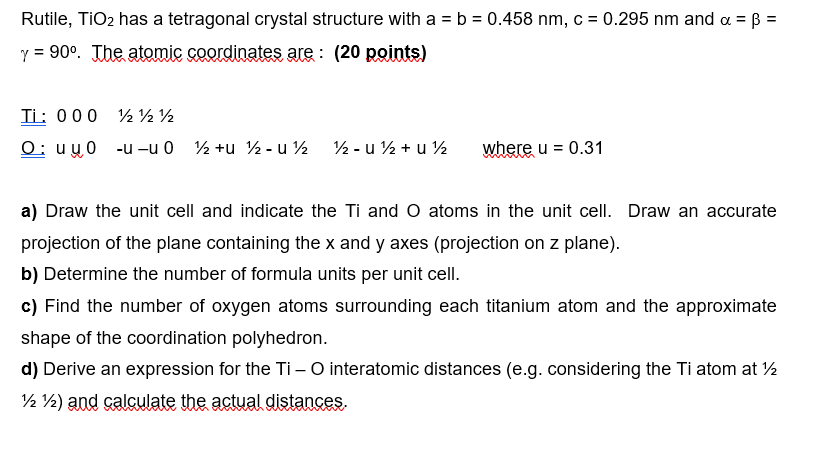
Rutile, TiO 2 has a tetragonal crystal structure with a = b = 0 . 4 5 8 Rutile, T i O 2 has a
Rutile, TiO has a tetragonal crystal structure with a b Rutile, has a tetragonal crystal structure with and
The atomic coordinates are : points
Ti:
: u u where
a Draw the unit cell and indicate the and atoms in the unit cell. Draw an accurate
projection of the plane containing the and axes projection on plane
b Determine the number of formula units per unit cell.
c Find the number of oxygen atoms surrounding each titanium atom and the approximate
shape of the coordination polyhedron.
d Derive an expression for the interatomic distances eg considering the atom at
and calculate the actual distances. nm
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


