Answered step by step
Verified Expert Solution
Question
1 Approved Answer
( s o l v e each part o n a paper and explain and make proper block diagram o n a paper sheet,you solved
each part a paper and explain and make proper block diagram a paper sheet,you solved previously assuming constant volume that wrong
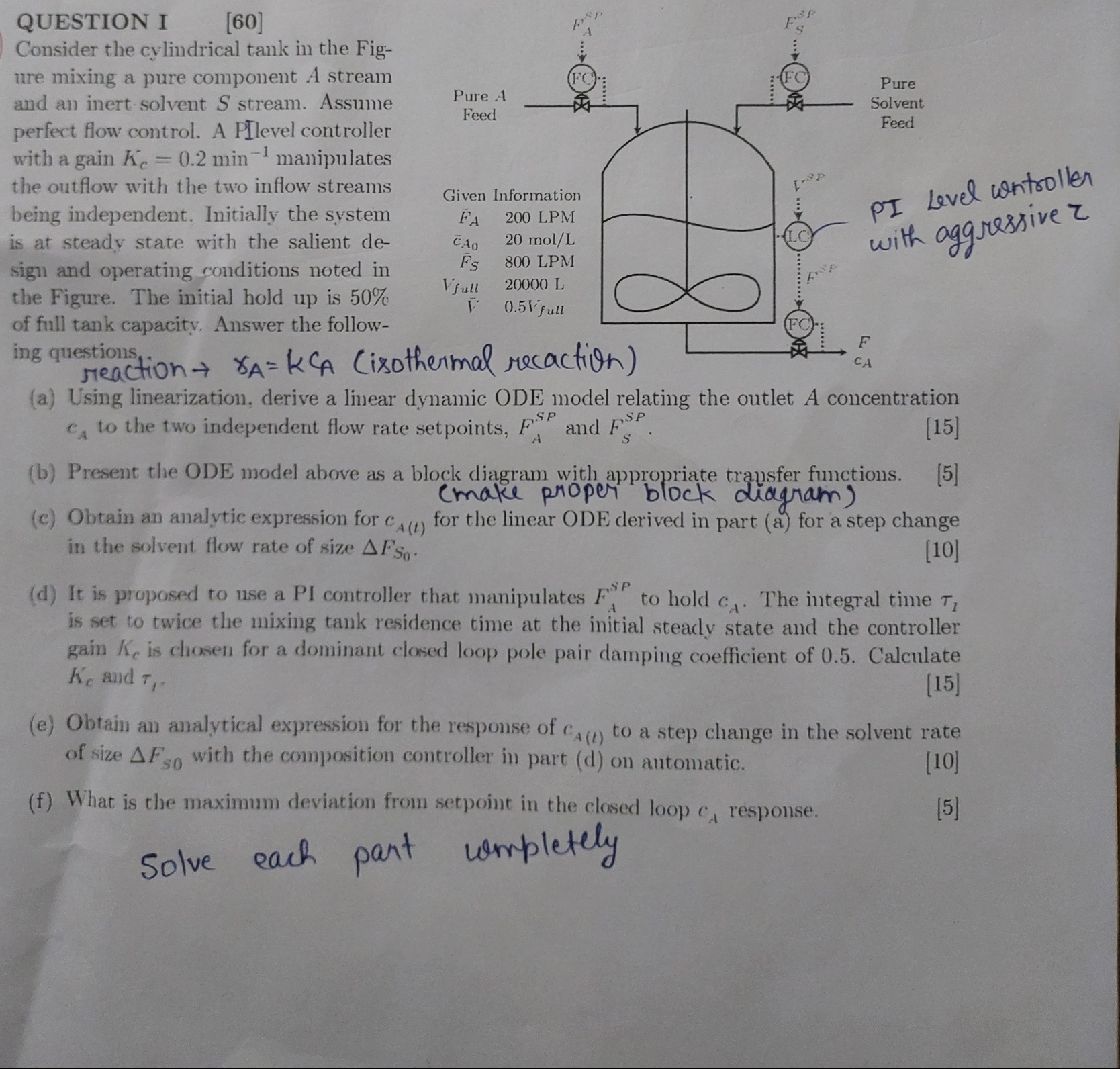
QUESTION I
Consider the cylindrical tank in the Fig
ure mixing a pure component A stream
and an inert solvent stream. Assume
perfect flow control. A PI level controller
with a gain with an aggressive manipulates
the outflow with the two inflow streams
being independent. Initially the system
is at steady state with the salient de
sign and operating conditions noted in
the Figure. The initial hold up is
of full tank capacity. Answer the follow
ing questions,
reaction isother reaction
volume not constant.
a Using linearization, derive a linear dynamic ODE model relating the outlet A concentration
to the two independent flow rate setpoints, and
b Present the ODE model above as a block diagram with appropriate transfer functions.
c Obtain an analytic expression for for the linear ODE derived in part a for a step change
in the solvent flow rate of size
d It is proposed to use a PI controller that manipulates to hold The integral time
is set to twice the mixing tank residence time at the initial steady state and the controller
gain is chosen for a dominant closed loop pole pair damping coefficient of Calculate
and
e Obtain an analytical expression for the response of to a step change in the solvent rate
of size with the composition controller in part d on antomatic.
f What is the maximum deviation from setpoint in the elosed loop response.
Solve each part commpletely and explain explain with proper reasoning
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


