Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Experimental Part 1 - Preparation of 4,7-Dihydroxyflavylium Chloride (DHF) A T B ago D C Figure 1. Known equilibria of the DHF cation (D)
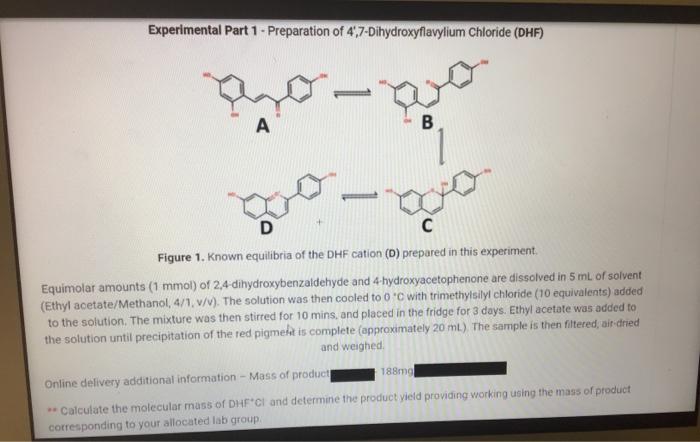
Experimental Part 1 - Preparation of 4,7-Dihydroxyflavylium Chloride (DHF) A T B ago D C Figure 1. Known equilibria of the DHF cation (D) prepared in this experiment. Equimolar amounts (1 mmol) of 2,4-dihydroxybenzaldehyde and 4-hydroxyacetophenone are dissolved in 5 mL of solvent (Ethyl acetate/Methanol, 4/1, v/v). The solution was then cooled to 0C with trimethylsilyl chloride (10 equivalents) added to the solution. The mixture was then stirred for 10 mins, and placed in the fridge for 3 days. Ethyl acetate was added to the solution until precipitation of the red pigment is complete (approximately 20 mL). The sample is then filtered, air-dried and weighed. 188mg Online delivery additional information - Mass of product **Calculate the molecular mass of DHF CI and determine the product yield providing working using the mass of product corresponding to your allocated lab group.
Step by Step Solution
★★★★★
3.38 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1
Answer HO HO MW 13812 H HO MW13615 HO Dzo HO HO MW 27404 OH 1 mmol of 24dihydroxybenza...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


