Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Saponification of ethyl acetate to produce sodium acetate and ethanol is a well - known and well - characterized reaction. Its kinetics are known to
Saponification of ethyl acetate to produce sodium acetate and ethanol is a wellknown and well
characterized reaction. Its kinetics are known to be overall second order, with the following
elementary reaction:
NaOH
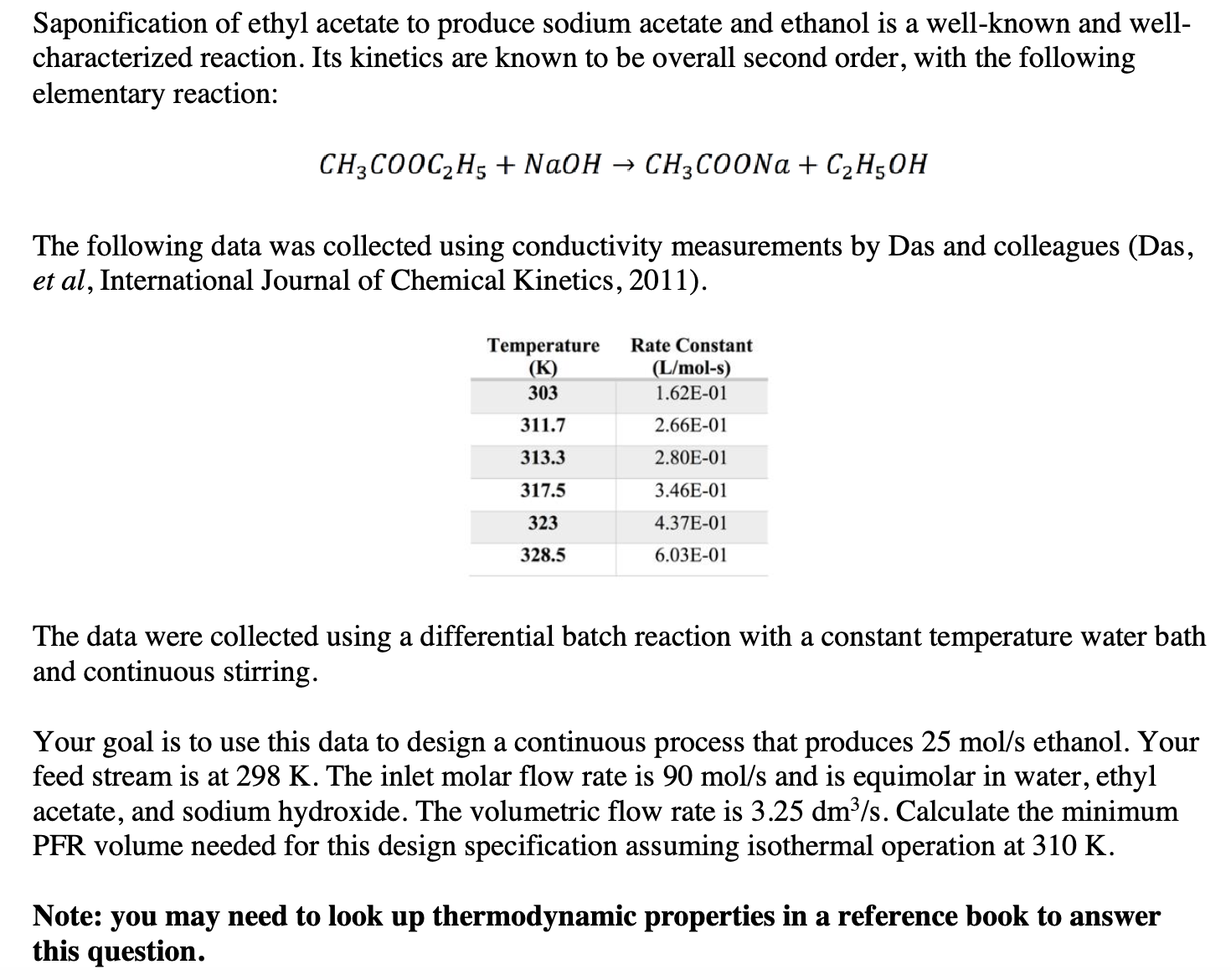
The following data was collected using conductivity measurements by Das and colleagues Das
et al International Journal of Chemical Kinetics,
The data were collected using a differential batch reaction with a constant temperature water bath
and continuous stirring.
Your goal is to use this data to design a continuous process that produces ethanol. Your
feed stream is at The inlet molar flow rate is and is equimolar in water, ethyl
acetate, and sodium hydroxide. The volumetric flow rate is Calculate the minimum
PFR volume needed for this design specification assuming isothermal operation at
Note: you may need to look up thermodynamic properties in a reference book to answer
this question.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


