Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A reaction profile (not to scale!) for the reaction 03 + 0 2 0 is shown below: E (KJ) 03+0 392 19 Reaction Coordinate
![A reaction profile (not to scale!) for the reaction [ mathrm{O}_{3}+mathrm{O} longrightarrow 2 mathrm{O}_{2} ] is shown](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2022/09/63198c52ea3c2_1662618703658.jpg)
![[ mathrm{O}_{3}+mathrm{O} rightarrow 2 mathrm{O}_{2} ] is shown below: [ E(k J) ] Which of the following are true? (S](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2022/09/63198c530efb2_1662618703844.jpg)
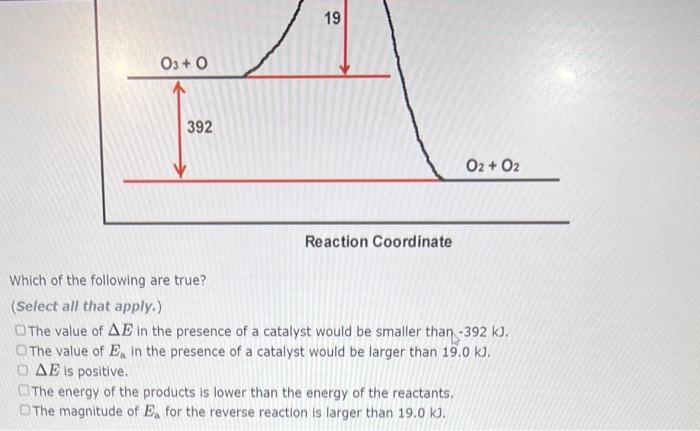
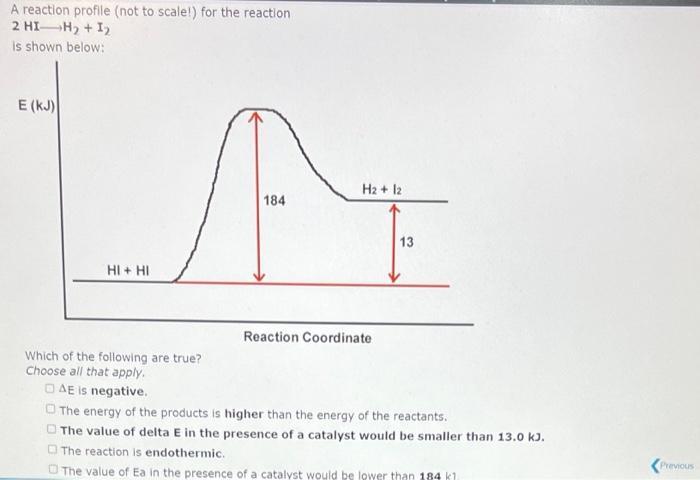
A reaction profile (not to scale!) for the reaction 03 + 0 2 0 is shown below: E (KJ) 03+0 392 19 Reaction Coordinate This reaction is (exothermic,endothermic) The value of the activation energy for this reaction is 02 + 02 kJ and the value of AE is kJ. A reaction profile (not to scale!) for the reaction 2 HI-H + 1 is shown below: E (KJ) HI+ HI Which of the following are true? Choose all that apply. AE is negative. 184 H2 + 12 Reaction Coordinate 13 The energy of the products is higher than the energy of the reactants. The value of delta E in the presence of a catalyst would be smaller than 13.0 kJ. The reaction is endothermic. The value of Ea in the presence of a catalyst would be lower than 184 kl Previous 03+0 20 is shown below: E (KJ) 03+0 392 19 Reaction Coordinate 02 + 02 Which of the following are true? (Select all that apply.) The value of AE in the presence of a catalyst would be smaller than -392 kJ. The value of E, in the presence of a catalyst would be larger than 19.0 kJ. DAE is positive. The energy Show Hint ducts is lower than the energy of the reactants. Pie 03+0 392 19 Reaction Coordinate 02 + 02 Which of the following are true? (Select all that apply.) The value of AE in the presence of a catalyst would be smaller than -392 kJ. The value of E, in the presence of a catalyst would be larger than 19.0 kJ. OAE is positive. The energy of the products is lower than the energy of the reactants. The magnitude of E, for the reverse reaction is larger than 19.0 kJ.
Step by Step Solution
★★★★★
3.47 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1
Q1 Q2 Q3 In the given energy profile 19 kj represents the activation energy Ea ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


