Question
Saturated water vapor initially at a pressure of 250 kPa and volume 0.01 is contained in a piston cylinder assembly. The water undergoes two
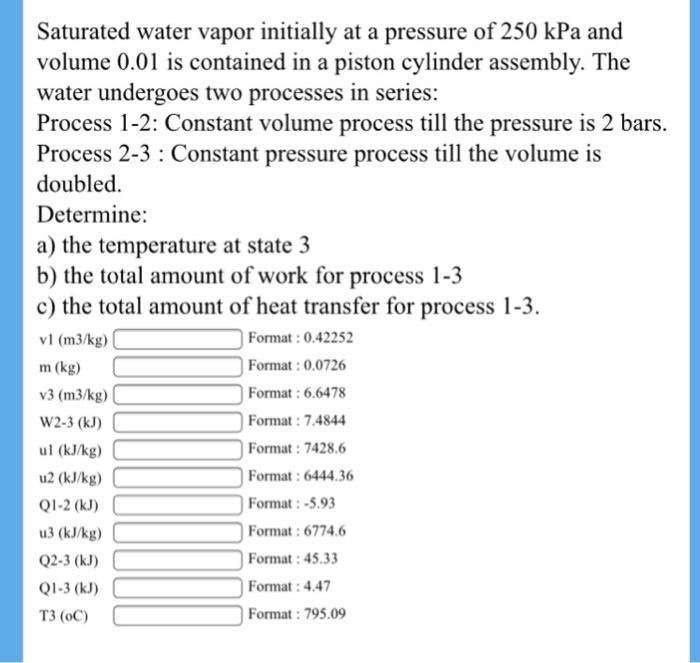
Saturated water vapor initially at a pressure of 250 kPa and volume 0.01 is contained in a piston cylinder assembly. The water undergoes two processes in series: Process 1-2: Constant volume process till the pressure is 2 bars. Process 2-3 : Constant pressure process till the volume is doubled. Determine: a) the temperature at state 3 b) the total amount of work for process 1-3 c) the total amount of heat transfer for process 1-3. vl (m3/kg) Format : 0.42252 m (kg) Format : 0.0726 v3 (m3/kg) Format : 6.6478 W2-3 (kJ) Format : 7.4844 ul (kJ/kg) Format : 7428.6 u2 (kJ/kg) Format : 6444.36 QI-2 (kJ) Format: -5.93 Format : 6774.6 Format : 45.33 u3 (kJ/kg) Q2-3 (kJ) QI-3 (kJ) Format: 4.47 3 () Format : 795.09
Step by Step Solution
3.32 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physics
Authors: Alan Giambattista, Betty Richardson, Robert Richardson
2nd edition
77339681, 978-0077339685
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



