Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Saturation vapor pressure equation for HCFC-123 Refrigerant(2,2 dichloro-1,1.1-trifloroethane) is given as: log 10 PA+B/T + ClogT+DT + E((F-T)/T)log (F-I) Curve fit values are given
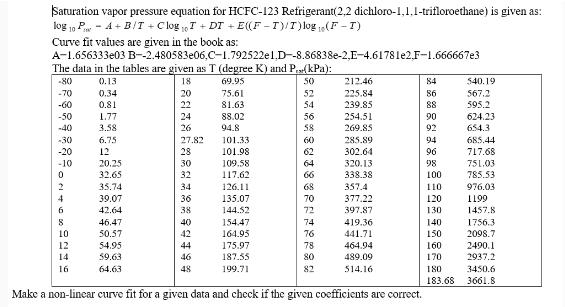
Saturation vapor pressure equation for HCFC-123 Refrigerant(2,2 dichloro-1,1.1-trifloroethane) is given as: log 10 PA+B/T + ClogT+DT + E((F-T)/T)log (F-I) Curve fit values are given in the book as: A-1.656333e03 The data in the tables are given as T (degree K) and P.(kPa): -80 0.13 18 69.95 50 -70 -60 -50 -40 -30 -20 -10 0 2 4 6 8 10 12 14 16 0.34 0.81 1.77 3.58 6.75 12 B--2.480583e06,C-1.792522e1.D--8.86838e-2,E-4.61781e2.F-1.666667e3 20.25 32.65 35.74 39.07 42.64 46.47 50.57 54.95 59.63 64.63 20 22 24 26 27.82 28 30 32 34 36 38 40 42 44 46 48 75.61 81.63 88.02 94.8 101.33 101.98 109.58 117.62 126.11 135.07 144.52 154.47 164.95 175.97 52 54 7 8 8 8 8 3 RFFERS 56 58 60 62 64 66 70 72 74 76 78 80 82 212.46 225.84 239.85 254.51 269.85 285.89 302.64 320.13 338.38 357.4 377.22 397,87 419.36 441.71 464.94 489.09 514.16 187.55 199.71 Make a non-linear curve fit for a given data and check if the given coefficients are correct. 84 86 88 90 92 94 96 98 100 110 120 130 140 150 160 170 180 183.68 $40.19 567.2 595.2 624.23 654.3 685.44 717.68 751.03 785.53 976.03 1199 1457.8 1756.3 2098.7 2490.1 2937.2 3450.6 3661.8
Step by Step Solution
★★★★★
3.39 Rating (165 Votes )
There are 3 Steps involved in it
Step: 1
Here are the steps to check the saturation vapor pressure equation and coefficients for HCFC123 refr...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


