Answered step by step
Verified Expert Solution
Question
1 Approved Answer
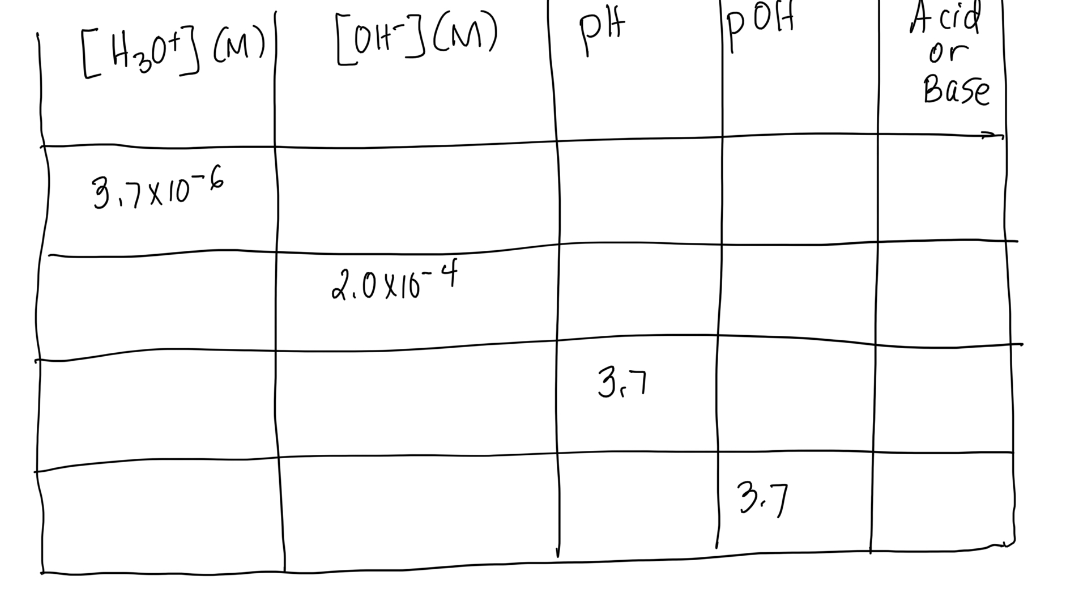
Scan and upload the file. Reproduce the table. Fill in the blanks. The formulas are: [H3O+][OH]=11014pH=log[H3O+][H3O+]=10pHpOH=log[OH][OH]=10pOHpH+pOH=14 begin{tabular}{|l|l|l|l|l|} {[H3O+](M)} & {[OH](M)} & PH & POH &

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


