Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Scientists use the pH scale to represent the level of acidity or alkalinity of a liquid. This is based on the molar concentration of
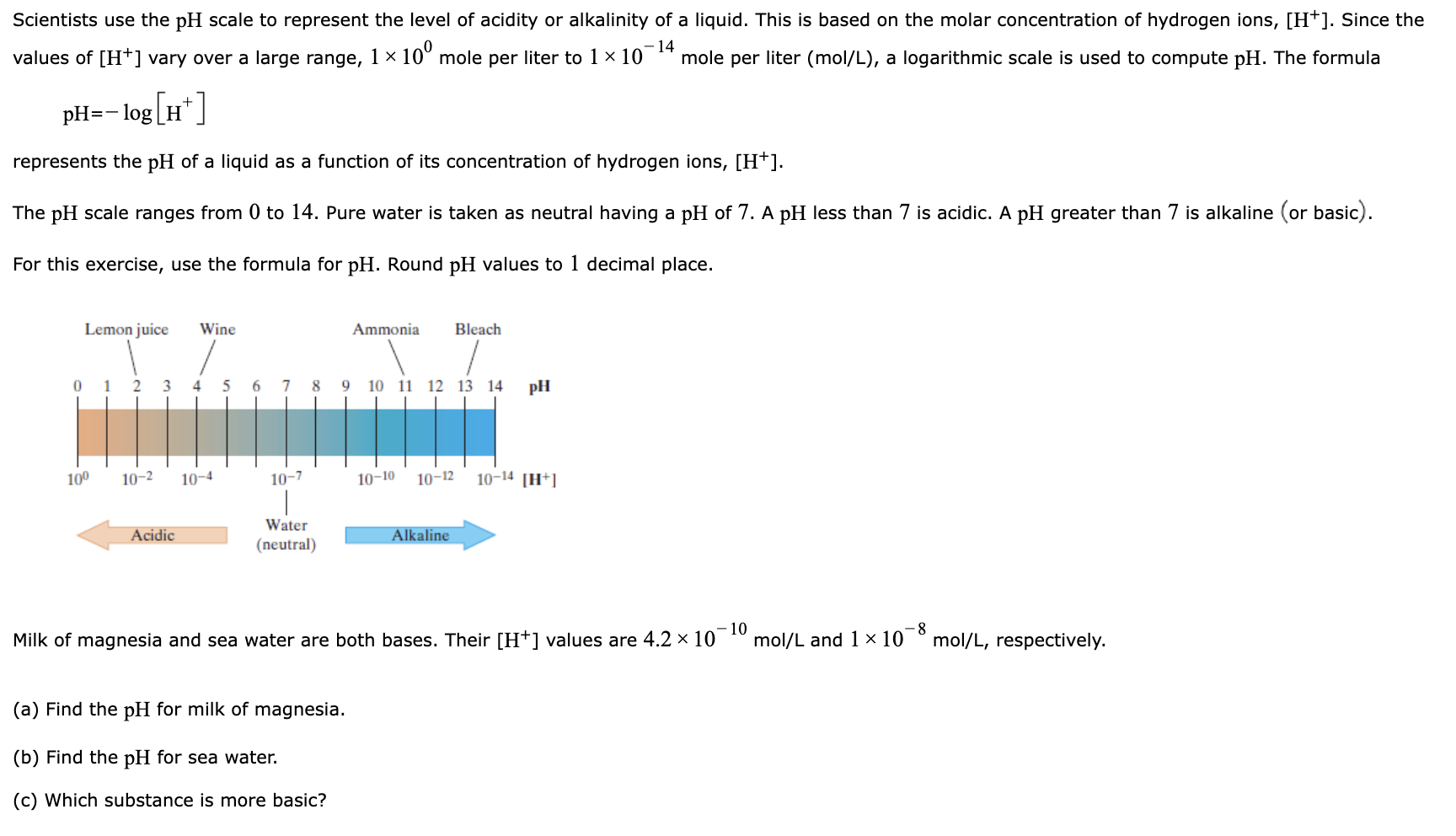
Scientists use the pH scale to represent the level of acidity or alkalinity of a liquid. This is based on the molar concentration of hydrogen ions, [H+]. Since the values of [H+] vary over a large range, 1 10 mole per liter to 1 10 mole per liter (mol/L), a logarithmic scale is used to compute pH. The formula -14 pH= log[H*] represents the pH of a liquid as a function of its concentration of hydrogen ions, [H+]. The pH scale ranges from 0 to 14. Pure water is taken as neutral having a pH of 7. A pH less than 7 is acidic. A pH greater than 7 is alkaline (or basic). For this exercise, use the formula for pH. Round pH values to 1 decimal place. Lemon juice Wine 0 12 3 4 5 6 7 8 9 10 11 12 13 14 10 10-2 Acidic 10-4 10-7 Water (neutral) Ammonia Bleach (a) Find the pH for milk of magnesia. (b) Find the pH for sea water. (c) Which substance is more basic? pH 10-10 10-12 10-14 [H+] Alkaline -10 Milk of magnesia and sea water are both bases. Their [H+] values are 4.2 10 mol/L and 1 10 -8 mol/L, respectively.
Step by Step Solution
★★★★★
3.36 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
a PH logH PH of milk of magnesia logH PH OF milk of magnesia PH of sea water l...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


