Answered step by step
Verified Expert Solution
Question
1 Approved Answer
second and third pictures are for the second and third question A student proposed the following mechanism for the gas phase reaction of fluorine with
second and third pictures are for the second and third question 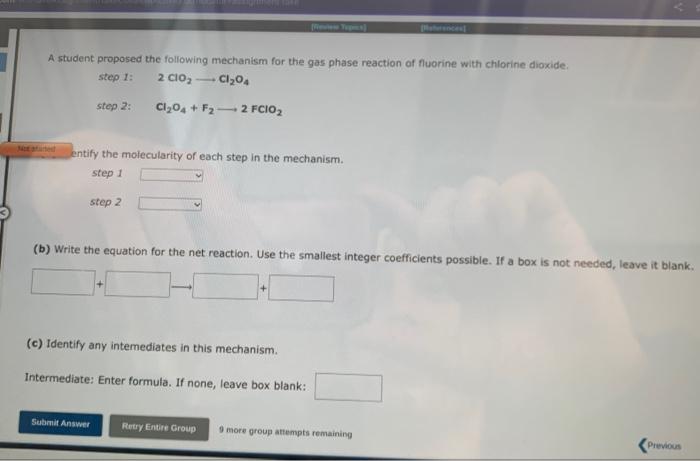
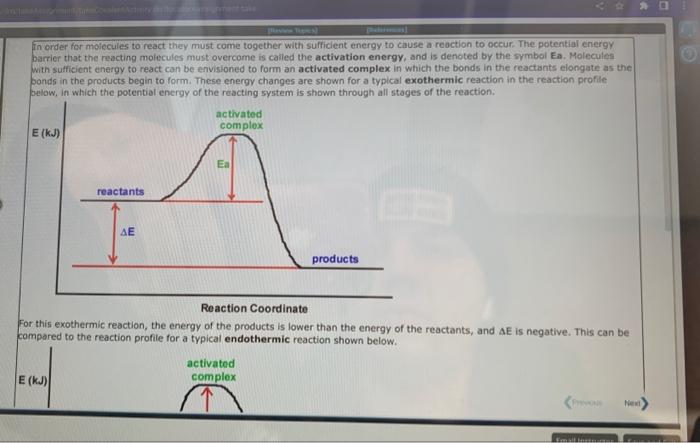
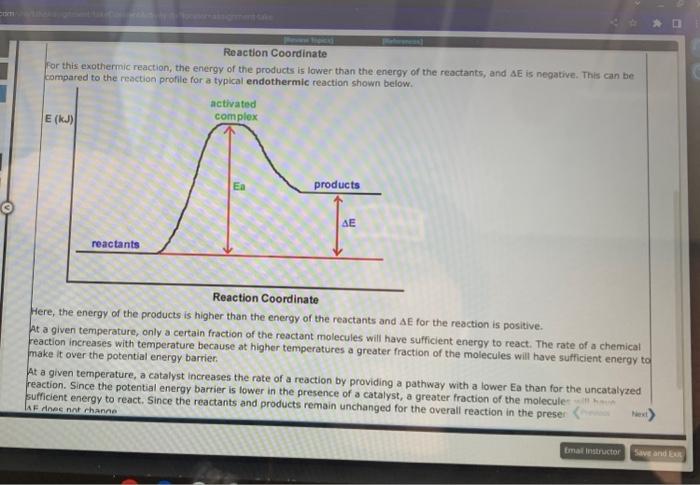
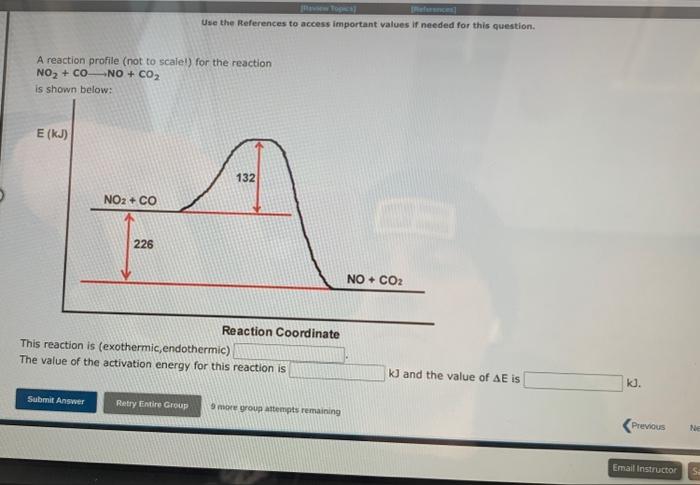
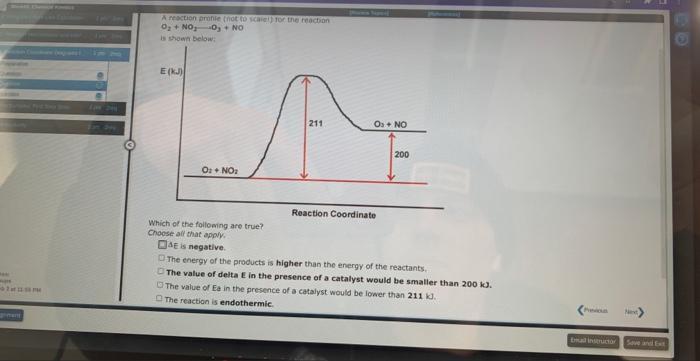
A student proposed the following mechanism for the gas phase reaction of fluorine with chlorine dioxide. step1:step2:2ClO2Cl2O4Cl2O4+F22FClO2 Mezatinet entify the molecularity of each step in the mechanism. step 1 step 2 (b) Write the equation for the net reaction. Use the smallest integer coefficients possible. If a box is not needed, leave it blank. (c) Identify any intemediates in this mechanism. Intermediate: Enter formula. If none, leave box blank: 9 more groug attempts remaining In order for moiecules to react they must come together with sufficient energy to cause a reoction to occur. The potential energy barrier that the reacting molecules must overcome is called the activation energy, and is denoted by the symbol Ea. Molecules with sufficient energy to react can be envisioned to form an activated complex in which the bonds in the reactants elongate as the bonds in the products begin to form. These energy changes are shown for a typical exothermic reaction in the reaction profile pelow, in which the potential energy of the reacting system is shown through all stages of the reaction. Reaction Coordinate For this exothermic reaction, the energy of the products is lower than the energy of the reactants, and AE is negative. This can be compared to the reaction profile for a typical endothermic reaction shown below. For this exothermic reaction, the energy of the products is lawer than the energy of the reactants, and AE is negative. This can be compared to the reaction profile for a typical endothermic reaction shown below. Reaction Coordinate Here, the energy of the products is higher than the energy of the reactants and AE for the reaction is positive. At a given temperature, only a certain fraction of the reactant molecules will have sufficient energy to react. The rate of a chemical reaction increases with temperature because at higher temperatures a greater fraction of the molecules will have sufficient energy to make it over the potential energy barrier. At a given temperature, a catalyst increases the rate of a reaction by providing a pathway with a lower Ea than for the uncatalyzed reaction. Since the potential energy barrier is lower in the presence of a catalyst, a greater fraction of the molecule: sufficient energy to Afk ines nat mhanne A reaction profile (not to scale!) for the reaction NO2+CONO+CO2 is shown below: Reaction Coordinate This reaction is (exothermic, endothermic) The value of the activation energy for this reaction is kJ and the value of AE is 9 mope group attempts remaining A meactuon prunile (not fs icaiei) for the maction. O2+NO2O3+NO is shawn below: Which of the following are true? Choose all that apory: Reaction Coordinate AE is negative. The energy of the products is higher than the energy of the reactants. The value of delta E in the presence of a catalyst would be 5 maller than 200kJ. The value of Ea in the presence of a catalyst would be lower than 211kJ. The reaction is endothermic 




Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


