Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Select. 1 = Buoyancy, diameter, density, mass, Volume Select 2 = Copper, Gold , Iron, Silver A large plece of jewelry has a mass of
Select. 1 = Buoyancy, diameter, density, mass, Volume
A large plece of jewelry has a mass of 134.6g. A graduated cylinder initially contains 48.0mL water. When the jewelry is submerged in the araduated cylinder, the total volume increases to 60.9mL. (a) Determine the density (in g/cm3) of this plece of jewelry. g/cm3 (b) Assuming that the jeweiry is made from only one substance, what substance is it likely to ben? Explain. (See the Densizes of Cammon Substances table.) The only metal with a close to the one caiculated for this piece of Jewelry is Select 2 = Copper, Gold , Iron, Silver 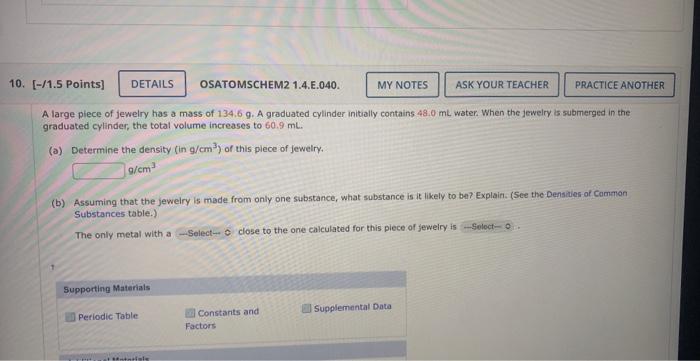

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


