Answered step by step
Verified Expert Solution
Question
1 Approved Answer
In very old stars, heavy metallic elements like uranium (U), osmium (Os), and iridium (Ir) are hard to come by. (Usually, these elements are
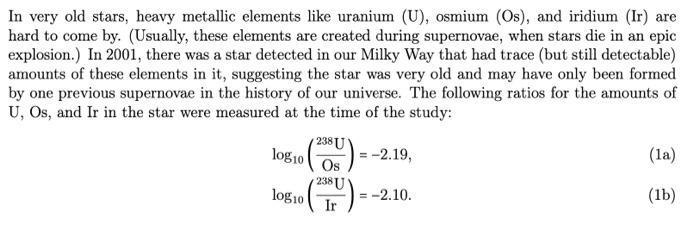
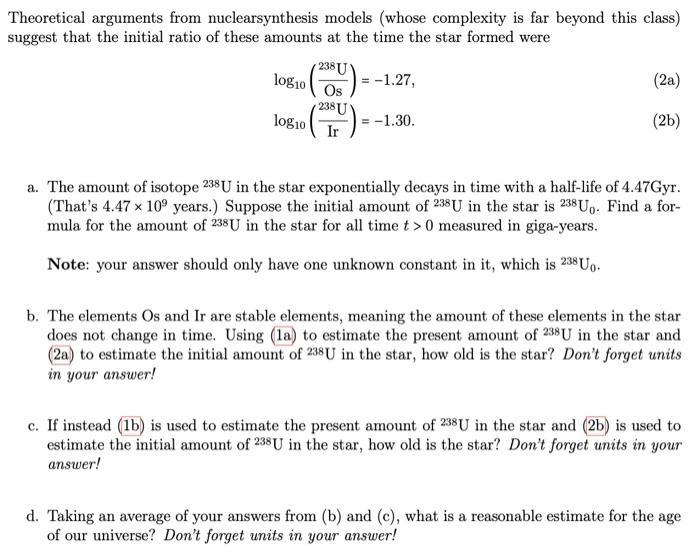
In very old stars, heavy metallic elements like uranium (U), osmium (Os), and iridium (Ir) are hard to come by. (Usually, these elements are created during supernovae, when stars die in an epic explosion.) In 2001, there was a star detected in our Milky Way that had trace (but still detectable) amounts of these elements in it, suggesting the star was very old and may have only been formed by one previous supernovae in the history of our universe. The following ratios for the amounts of U, Os, and Ir in the star were measured at the time of the study: 238 U (05) = -2.19, log10 log10 2381 18 U Ir = = -2.10. (la) (lb) Theoretical arguments from nuclearsynthesis models (whose complexity is far beyond this class) suggest that the initial ratio of these amounts at the time the star formed were 2381 (200) = -1.27, Os = -1.30. log10 log10 2381 Ir (2a) (2b) a. The amount of isotope 238 U in the star exponentially decays in time with a half-life of 4.47Gyr. (That's 4.47 x 10 years.) Suppose the initial amount of 238 U in the star is 238 Uo. Find a for- mula for the amount of 238U in the star for all time t> 0 measured in giga-years. Note: your answer should only have one unknown constant in it, which is 238 Uo. b. The elements Os and Ir are stable elements, meaning the amount of these elements in the star does not change in time. Using (la) to estimate the present amount of 238 U in the star and (2a) to estimate the initial amount of 238U in the star, how old is the star? Don't forget units in your answer! c. If instead (1b) is used to estimate the present amount of 238 U in the star and (2b) is used to estimate the initial amount of 238 U in the star, how old is the star? Don't forget units in your answer! d. Taking an average of your answers from (b) and (c), what is a reasonable estimate for the age of our universe? Don't forget units in your answer!
Step by Step Solution
★★★★★
3.47 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


