Answered step by step
Verified Expert Solution
Question
1 Approved Answer
General Chemistry 4th Edition McQuarrie Rock Gallogly Initial-rate data at a certain temperature is given in the table for the reaction SOCl(g) SO(g) +
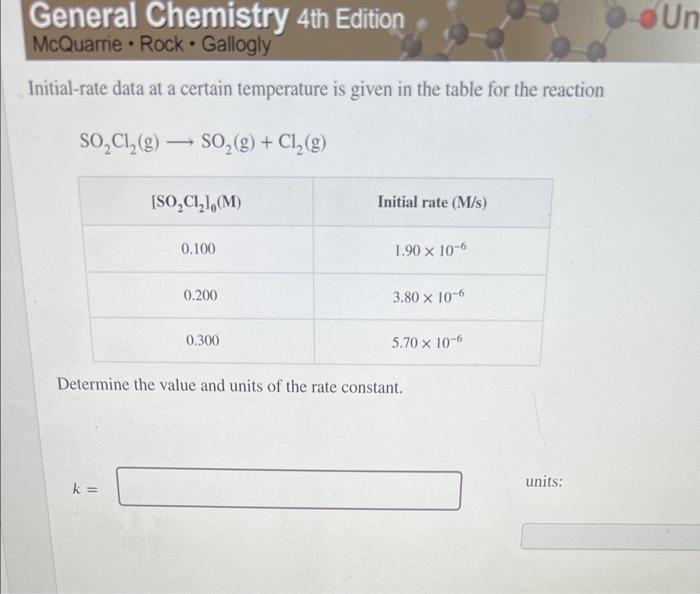
General Chemistry 4th Edition McQuarrie Rock Gallogly Initial-rate data at a certain temperature is given in the table for the reaction SOCl(g) SO(g) + Cl(g) [SOCl], (M) k= 0.100 0.200 0.300 Initial rate (M/s) 1.90 x 10-6 3.80 x 10-6 5.70 x 10-6 Determine the value and units of the rate constant. units: Un
Step by Step Solution
★★★★★
3.39 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
Step 1 Given reaction Step 2 N303 9 NOg NO2 g Rate ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
635d71bd4c677_175672.pdf
180 KBs PDF File
635d71bd4c677_175672.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


