Question
2.2. A sample of a diatomic ideal gas has pressure P and volume V. When the gas is warmed, its pressure triple, volume triple,
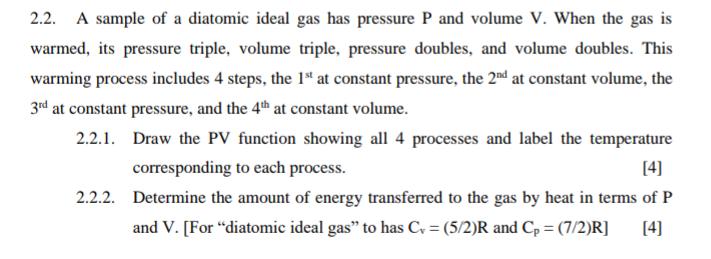
2.2. A sample of a diatomic ideal gas has pressure P and volume V. When the gas is warmed, its pressure triple, volume triple, pressure doubles, and volume doubles. This warming process includes 4 steps, the 1st at constant pressure, the 2nd at constant volume, the 3rd at constant pressure, and the 4th at constant volume. 2.2.1. Draw the PV function showing all 4 processes and label the temperature corresponding to each process. [4] 2.2.2. Determine the amount of energy transferred to the gas by heat in terms of P and V. [For "diatomic ideal gas" to has C = (5/2)R and Cp = (7/2)R] [4]
Step by Step Solution
3.36 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
221 PV diagram 6P 3P P P A V Q nCpAT BRAT 3V 222 heat s...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Auditing and Assurance Services A Systematic Approach
Authors: William Messier, Steven Glover, Douglas Prawitt
9th edition
1308361491, 77862333, 978-1259248290, 9780077862336, 1259162346, 978-1259162343
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



