Question
3. [Properties of a Diatomic Ideal Gas]: Consider a diatomic ideal gas of fixed N, V, and T for which you can represent the
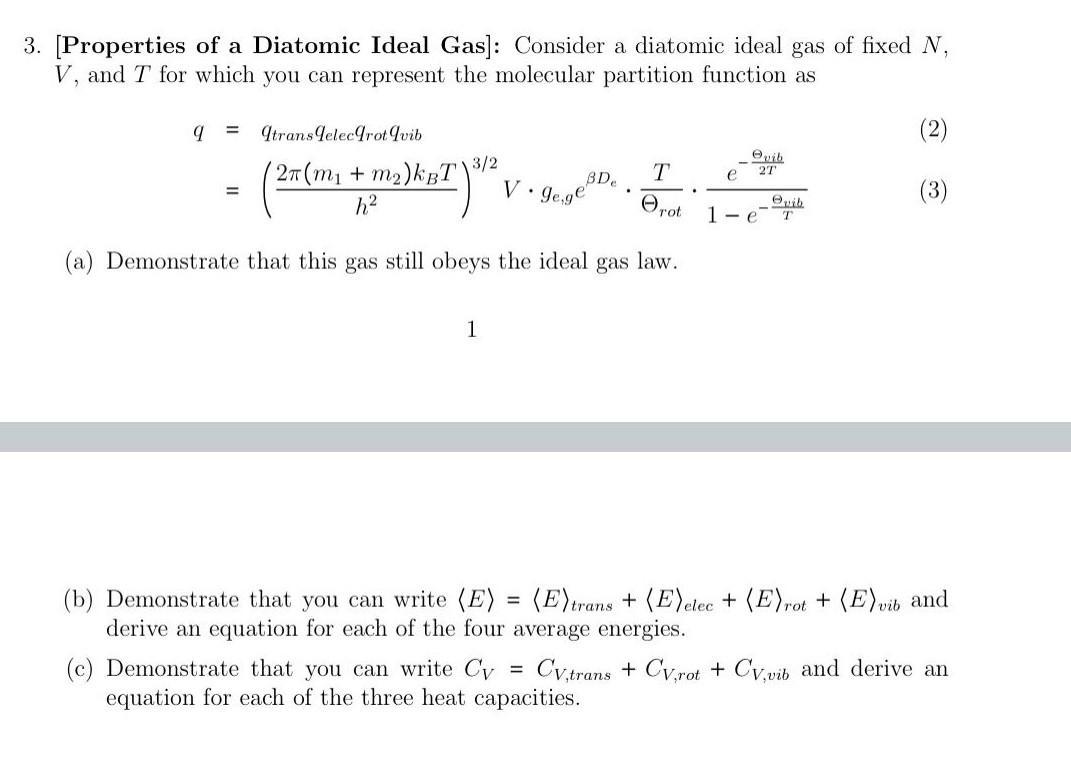
3. [Properties of a Diatomic Ideal Gas]: Consider a diatomic ideal gas of fixed N, V, and T for which you can represent the molecular partition function as 9 = qtransdelecrot qvib = 3/2 (27 (m + m)kut) 2) KBT h 1 T BDe V. gege (a) Demonstrate that this gas still obeys the ideal gas . (c) Demonstrate that you can write Cy equation for each of the three heat capacities. rot law. e 27 1-e T (3) (b) Demonstrate that you can write (E) = (E) trans + (E)elec + (E)rot + (E)vib and derive an equation for each of the four average energies. Cv,trans + CV,rot + Cv,vib and derive an
Step by Step Solution
3.44 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1
Demonstrate that this gas still obeys the ideal gas law V gege BDe T Prot 3 Properties of a Diatomic Ideal Gas Consider a diatomic ideal gas of fixed N V and T for which you can represent the molecula...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Business Law Text and Cases
Authors: Kenneth W. Clarkson, Roger LeRoy Miller, Gaylord A. Jentz, F
11th Edition
324655223, 978-0324655223
Students also viewed these Law questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



