Answered step by step
Verified Expert Solution
Question
1 Approved Answer
show all working need asap The diffusion coefficient of hydrogen (H2) in air is D=0.63104m2/s at 293K and 1atm. By comparison, predict the diffusion coefficient
show all working need asap 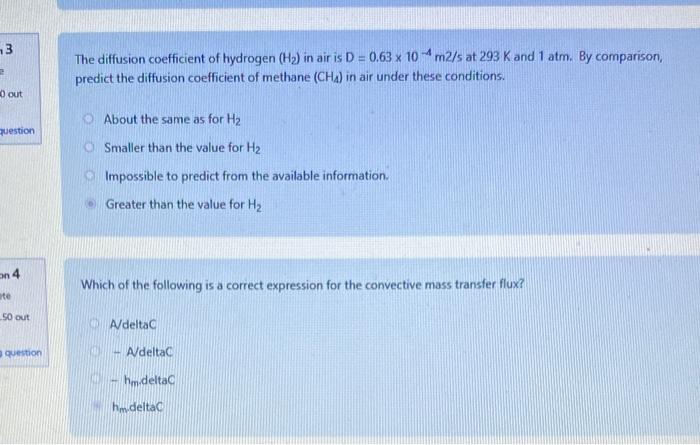

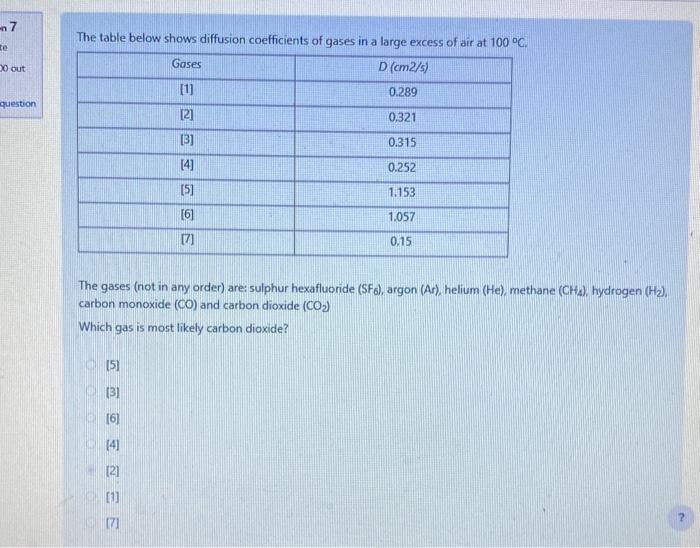

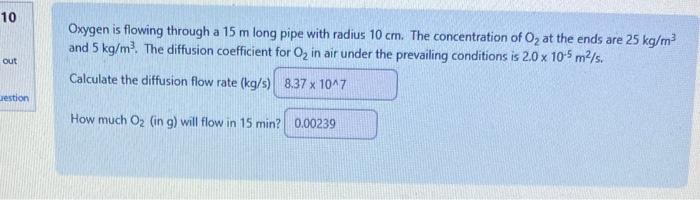 The diffusion coefficient of hydrogen (H2) in air is D=0.63104m2/s at 293K and 1atm. By comparison, predict the diffusion coefficient of methane (CH4) in air under these conditions. About the same as for H2 Smaller than the value for H2 Impossible to predict from the available information. Greater than the value for H2 Which of the following is a correct expression for the convective mass transfer flux? AdeltaC - AdeltaC hm deltaC Which of the following is true of convective mass transfer? It is analogous to heat conduction It is slower than molecular diffusion It requires a concentration gradient It involves a phase boundary The table below shows diffusion coefficients of gases in a large excess of air at 100C. The gases (not in any order) are: sulphur hexafluoride (SF6), argon (Ar), helium (He), methane ( CH4 ), hydrogen (H2 ), carbon monoxide (CO) and carbon dioxide (CO2) Which gas is most likely carbon dioxide? [5) [3] [6] [4] [2] [] [7] The table below shows diffusion coefficients of gases in a large excess of air at 100C, The gases (not in any order) are: sulphur hexafluoride (SFo), argon (Ar), helium (He), methane (CH4), hydrogen (H2), carbon monoxide (CO) and carbon dioxide (CO2) Which gas is most likely sulphur hexafluoride? [1] [7] (3) [6] [2] [5] Oxygen is flowing through a 15m long pipe with radius 10cm. The concentration of O2 at the ends are 25kg/m3 and 5kg/m3. The diffusion coefficient for O2 in air under the prevailing conditions is 2.0105m2/s. Calculate the diffusion flow rate (kg/s) How much O2 (in g ) will flow in 15min ? The diffusion coefficient of hydrogen (H2) in air is D=0.63104m2/s at 293K and 1atm. By comparison, predict the diffusion coefficient of methane (CH4) in air under these conditions. About the same as for H2 Smaller than the value for H2 Impossible to predict from the available information. Greater than the value for H2 Which of the following is a correct expression for the convective mass transfer flux? AdeltaC - AdeltaC hm deltaC Which of the following is true of convective mass transfer? It is analogous to heat conduction It is slower than molecular diffusion It requires a concentration gradient It involves a phase boundary The table below shows diffusion coefficients of gases in a large excess of air at 100C. The gases (not in any order) are: sulphur hexafluoride (SF6), argon (Ar), helium (He), methane ( CH4 ), hydrogen (H2 ), carbon monoxide (CO) and carbon dioxide (CO2) Which gas is most likely carbon dioxide? [5) [3] [6] [4] [2] [] [7] The table below shows diffusion coefficients of gases in a large excess of air at 100C, The gases (not in any order) are: sulphur hexafluoride (SFo), argon (Ar), helium (He), methane (CH4), hydrogen (H2), carbon monoxide (CO) and carbon dioxide (CO2) Which gas is most likely sulphur hexafluoride? [1] [7] (3) [6] [2] [5] Oxygen is flowing through a 15m long pipe with radius 10cm. The concentration of O2 at the ends are 25kg/m3 and 5kg/m3. The diffusion coefficient for O2 in air under the prevailing conditions is 2.0105m2/s. Calculate the diffusion flow rate (kg/s) How much O2 (in g ) will flow in 15min
The diffusion coefficient of hydrogen (H2) in air is D=0.63104m2/s at 293K and 1atm. By comparison, predict the diffusion coefficient of methane (CH4) in air under these conditions. About the same as for H2 Smaller than the value for H2 Impossible to predict from the available information. Greater than the value for H2 Which of the following is a correct expression for the convective mass transfer flux? AdeltaC - AdeltaC hm deltaC Which of the following is true of convective mass transfer? It is analogous to heat conduction It is slower than molecular diffusion It requires a concentration gradient It involves a phase boundary The table below shows diffusion coefficients of gases in a large excess of air at 100C. The gases (not in any order) are: sulphur hexafluoride (SF6), argon (Ar), helium (He), methane ( CH4 ), hydrogen (H2 ), carbon monoxide (CO) and carbon dioxide (CO2) Which gas is most likely carbon dioxide? [5) [3] [6] [4] [2] [] [7] The table below shows diffusion coefficients of gases in a large excess of air at 100C, The gases (not in any order) are: sulphur hexafluoride (SFo), argon (Ar), helium (He), methane (CH4), hydrogen (H2), carbon monoxide (CO) and carbon dioxide (CO2) Which gas is most likely sulphur hexafluoride? [1] [7] (3) [6] [2] [5] Oxygen is flowing through a 15m long pipe with radius 10cm. The concentration of O2 at the ends are 25kg/m3 and 5kg/m3. The diffusion coefficient for O2 in air under the prevailing conditions is 2.0105m2/s. Calculate the diffusion flow rate (kg/s) How much O2 (in g ) will flow in 15min ? The diffusion coefficient of hydrogen (H2) in air is D=0.63104m2/s at 293K and 1atm. By comparison, predict the diffusion coefficient of methane (CH4) in air under these conditions. About the same as for H2 Smaller than the value for H2 Impossible to predict from the available information. Greater than the value for H2 Which of the following is a correct expression for the convective mass transfer flux? AdeltaC - AdeltaC hm deltaC Which of the following is true of convective mass transfer? It is analogous to heat conduction It is slower than molecular diffusion It requires a concentration gradient It involves a phase boundary The table below shows diffusion coefficients of gases in a large excess of air at 100C. The gases (not in any order) are: sulphur hexafluoride (SF6), argon (Ar), helium (He), methane ( CH4 ), hydrogen (H2 ), carbon monoxide (CO) and carbon dioxide (CO2) Which gas is most likely carbon dioxide? [5) [3] [6] [4] [2] [] [7] The table below shows diffusion coefficients of gases in a large excess of air at 100C, The gases (not in any order) are: sulphur hexafluoride (SFo), argon (Ar), helium (He), methane (CH4), hydrogen (H2), carbon monoxide (CO) and carbon dioxide (CO2) Which gas is most likely sulphur hexafluoride? [1] [7] (3) [6] [2] [5] Oxygen is flowing through a 15m long pipe with radius 10cm. The concentration of O2 at the ends are 25kg/m3 and 5kg/m3. The diffusion coefficient for O2 in air under the prevailing conditions is 2.0105m2/s. Calculate the diffusion flow rate (kg/s) How much O2 (in g ) will flow in 15min
need asap 









Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


