Question
Show all your work and keep significant figures in mind through the problem. Thank you in advance. On the equation below please disregard the :
Show all your work and keep significant figures in mind through the problem. Thank you in advance. On the equation below please disregard the : and .
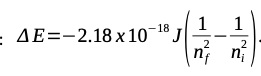

- AE=-2.18 x 10-18 J 1 2 nf 2 n a. Calculate the energy required to ionize a hydrogen atom using the Rydberg Equation above. b. What wavelength of light would be required to ionize a hydrogen atom? C. In what region of the electromagnetic spectrum does the light emission occur?
Step by Step Solution
There are 3 Steps involved in it
Step: 1
a The energy required to ionize a hydrogen atom using the Rydberg equation can be ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Essentials Of Statistics For The Behavioral Sciences
Authors: Frederick J Gravetter, Larry B. Wallnau
8th Edition
1133956572, 978-1133956570
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



