Question
Find the percent compositions of all of the elements in the following compounds: 1) CuBr2 Cu: Br: 2) NaOH Na: O: H: 3) (NH4)2S
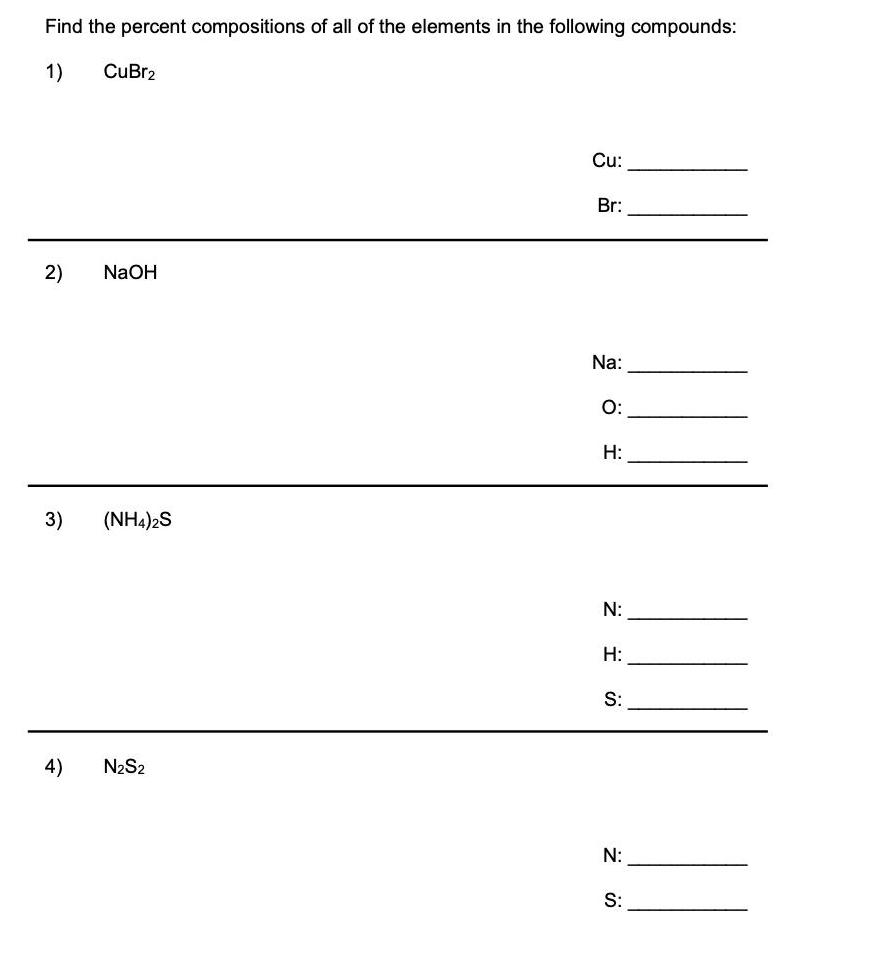
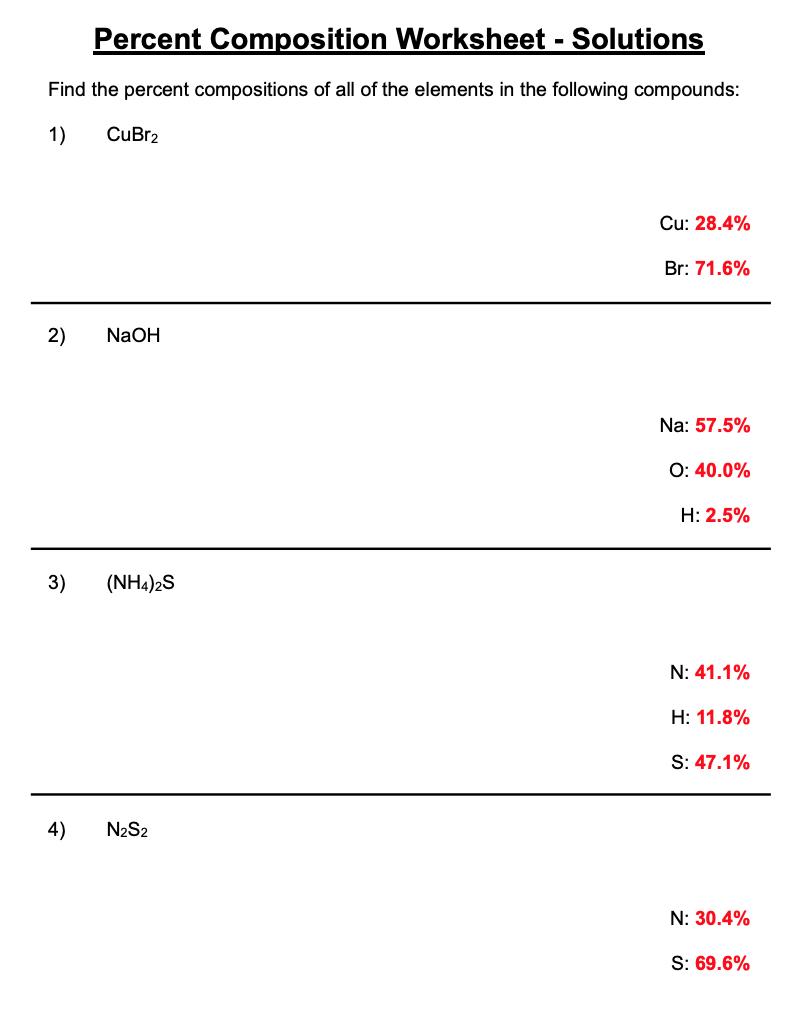
Find the percent compositions of all of the elements in the following compounds: 1) CuBr2 Cu: Br: 2) NaOH Na: O: H: 3) (NH4)2S N: H: S: 4) N2S2 N: S: Percent Composition Worksheet Solutions Find the percent compositions of all of the elements in the following compounds: 1) CuBr2 Cu: 28.4% Br: 71.6% 2) NaOH Na: 57.5% O: 40.0% H: 2.5% 3) (NH4)2S N: 41.1% H: 11.8% S: 47.1% 4) N2S2 N: 30.4% S: 69.6%
Step by Step Solution
3.51 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1
1 CuBr2 atomic mass of Cu 635 g atomic mass of Br 80 g total mass of the compound ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Differential Equations and Linear Algebra
Authors: Jerry Farlow, James E. Hall, Jean Marie McDill, Beverly H. West
2nd edition
131860615, 978-0131860612
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



