Answered step by step
Verified Expert Solution
Question
1 Approved Answer
show how to solve 1.98gH is allowed to rooct with 9.80gN2 producing 1.31gNH. The Haber-Bosch process is a very important Part A industriat process. In
show how to solve 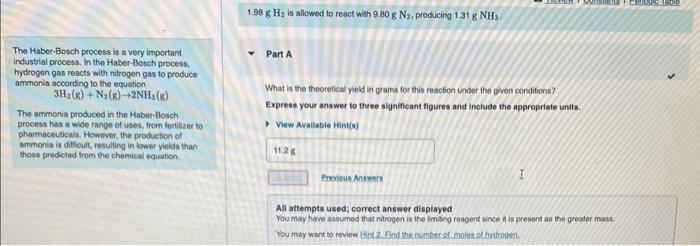
1.98gH is allowed to rooct with 9.80gN2 producing 1.31gNH. The Haber-Bosch process is a very important Part A industriat process. In the Haber-Bosch process, tydrogen gas reacts with nitrogon gas to produce ammonia according to the equation 3H2(g)+N2(g)2NH3(g) What is the theoretical yield in grams for this reaction under the given conditions? The ammonia produced in the Haber-Bosch process has a wide range of uses, from lecilizer to Express your answer to three significant figures and include the appropriate unifs. pharmaceuticals. Hewever, the peoducticn of ammonia is difficuit, resultirg in lower yields than those predieted from the chemical equation. All attempts used; correct answer displayed You may have asgumed that nitrogen is the imaing reagent since it is presont as the greater mask: You may want to reviow tiol 2. Find the in mber of moles of hydoogen 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


