Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Show that e defines the direction of the symmetry axis for h, e defines the direction of the symmetry axis for h, e defines
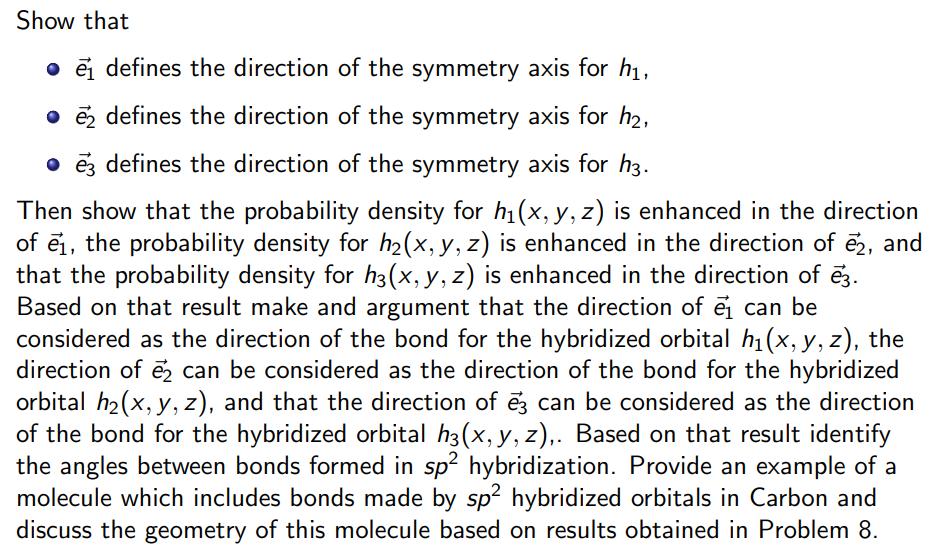
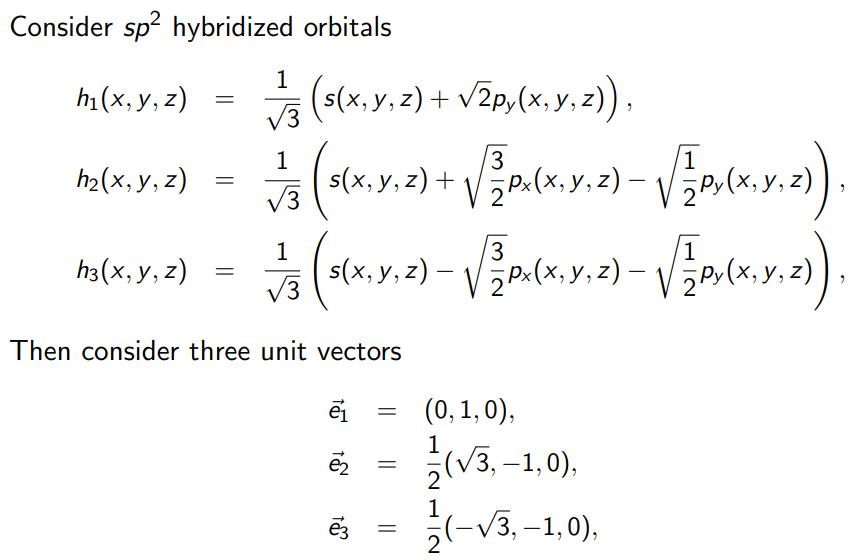
Show that e defines the direction of the symmetry axis for h, e defines the direction of the symmetry axis for h, e defines the direction of the symmetry axis for h3. Then show that the probability density for h(x, y, z) is enhanced in the direction of e, the probability density for h(x, y, z) is enhanced in the direction of 2, and that the probability density for h3(x, y, z) is enhanced in the direction of 23. Based on that result make and argument that the direction of can be considered as the direction of the bond for the hybridized orbital h(x, y, z), the direction of 2 can be considered as the direction of the bond for the hybridized orbital h(x, y, z), and that the direction of 3 can be considered as the direction of the bond for the hybridized orbital h3(x, y, z),. Based on that result identify the angles between bonds formed in sp hybridization. Provide an example of a molecule which includes bonds made by sp2 hybridized orbitals in Carbon and discuss the geometry of this molecule based on results obtained in Problem 8. Consider sp hybridized h(x, y, z) h(x, y, z) h3(x, y, z) = = = orbitals 1 (s(x, y, z) + 2py(x, y, z)), 3 3 1 1/13 (5(X,Y,Z) + P.(X, Y, 2) [P. (X.Y,.2)). x(x, y, - 3 1/13 s(x,y,z). Then consider three unit vectors e t t = || || 3 1 - P. (X,Y,Z) - PV(X, Y, 2)). z) (0, 1,0), 1 NIT NI (3,-1,0), (-3,-1,0),
Step by Step Solution
★★★★★
3.52 Rating (145 Votes )
There are 3 Steps involved in it
Step: 1
To show that e e and e define the directions of symmetry axes for h h and h respectively we need to calculate the dot product between each hybridized ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


