Answered step by step
Verified Expert Solution
Question
1 Approved Answer
show the graph to find the number of theoritical stages Oil is to be extracted from 10,000lb of granulated halibut livers, based on oil free
show the graph to find the number of theoritical stages 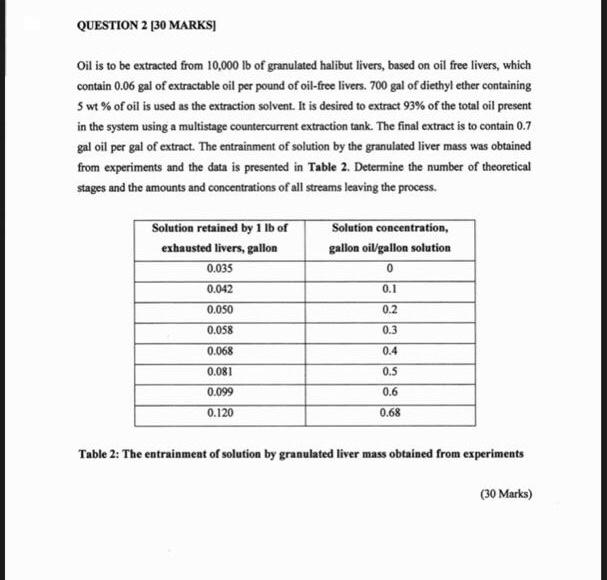
Oil is to be extracted from 10,000lb of granulated halibut livers, based on oil free livers, which contain 0.06 gal of extractable oil per pound of oil-free livers. 700gal of diethyl ether containing 5wt% of oil is used as the extraction solvent. It is desired to extract 93% of the total oil present in the system using a multistage countercurrent extraction tank. The final extract is to contain 0.7 gal oil per gal of extract. The entrainment of solution by the granulated liver mass was obtained from experiments and the data is presented in Table 2. Determine the number of theoretical stages and the amounts and concentrations of all streams leaving the process. Table 2: The entrainment of solution by granulated liver mass obtained from experiments Oil is to be extracted from 10,000lb of granulated halibut livers, based on oil free livers, which contain 0.06 gal of extractable oil per pound of oil-free livers. 700gal of diethyl ether containing 5wt% of oil is used as the extraction solvent. It is desired to extract 93% of the total oil present in the system using a multistage countercurrent extraction tank. The final extract is to contain 0.7 gal oil per gal of extract. The entrainment of solution by the granulated liver mass was obtained from experiments and the data is presented in Table 2. Determine the number of theoretical stages and the amounts and concentrations of all streams leaving the process. Table 2: The entrainment of solution by granulated liver mass obtained from experiments 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


