Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. Determine the pH of the solution that results from mixing 0.40 g of solid NaOH (40.00 g/mol) with 50.0 mL of a 0.10
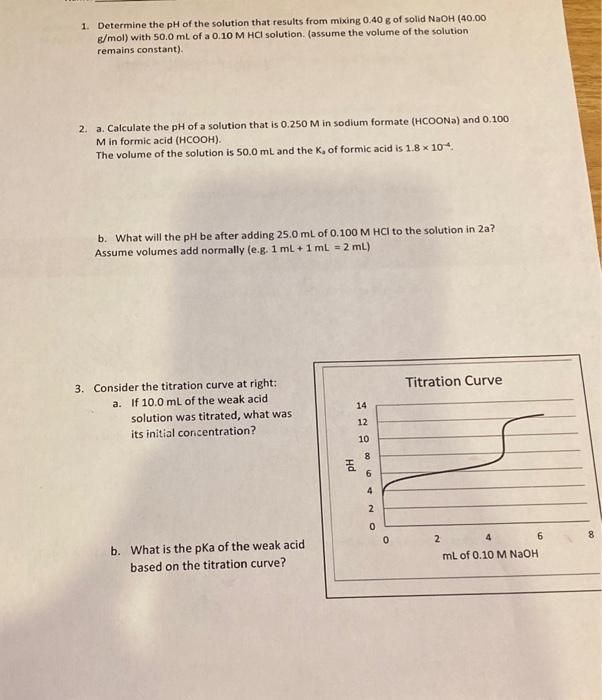

1. Determine the pH of the solution that results from mixing 0.40 g of solid NaOH (40.00 g/mol) with 50.0 mL of a 0.10 M HCl solution. (assume the volume of the solution remains constant). 2. a. Calculate the pH of a solution that is 0.250 M in sodium formate (HCOONa) and 0.100 M in formic acid (HCOOH). The volume of the solution is 50.0 mL and the K, of formic acid is 1.8 x 10. b. What will the pH be after adding 25.0 mL of 0.100 M HCI to the solution in 2a? Assume volumes add normally (e.g. 1 ml + 1 mL = 2 ml) 3. Consider the titration curve at right: a. If 10.0 mL of the weak acid solution was titrated, what was its initial concentration? b. What is the pka of the weak acid based on the titration curve? Hd 14 12 10 8 ON 000 Titration Curve 2 4 6 mL of 0.10 M NaOH 4. A 25.0 mL sample of a weak acid with a K, of 1.0 x 10 is titrated with 0.10 M NaOH. Based on the equivalence point volume, the concentration of the weak acid is determined to be 0.15 M. a. What volume of NaOH was required to reach the equivalence point? b. Calculate the pH of the solution before any NaOH has been added. c. Calculate the pH of the solution after 25.0 mL of NaOH has been added. d. Calculate the pH of the solution at the equivalence point. 5. The Ksp of silver sulfite, AgSO, is 1.5x 10-4 at 25 C. a. How many grams of silver sulfite are dissolved in 250. mL of a saturated solution at this temperature? b. What is the molar solubility of silver sulfite in a solution that contains 0.10 M AgNO?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


