Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Similar questions have been posted but none of the answers fully answer all parts of the problem. Specifically, make sure to include the reasoning for
Similar questions have been posted but none of the answers fully answer all parts of the problem. Specifically, make sure to include the reasoning for part b and the friction coefficient calculation for part c in your answer. thanks!
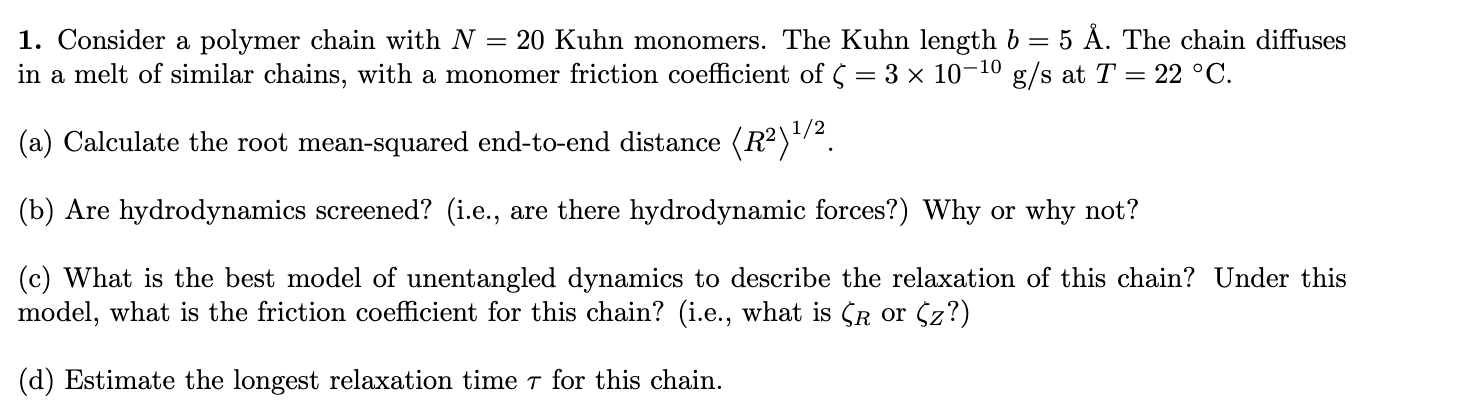
1. Consider a polymer chain with N=20 Kuhn monomers. The Kuhn length b=5A. The chain diffuses in a melt of similar chains, with a monomer friction coefficient of =31010g/s at T=22C. (a) Calculate the root mean-squared end-to-end distance R21/2. (b) Are hydrodynamics screened? (i.e., are there hydrodynamic forces?) Why or why not? (c) What is the best model of unentangled dynamics to describe the relaxation of this chain? Under this model, what is the friction coefficient for this chain? (i.e., what is R or Z ?) (d) Estimate the longest relaxation time for this chain. 1. Consider a polymer chain with N=20 Kuhn monomers. The Kuhn length b=5A. The chain diffuses in a melt of similar chains, with a monomer friction coefficient of =31010g/s at T=22C. (a) Calculate the root mean-squared end-to-end distance R21/2. (b) Are hydrodynamics screened? (i.e., are there hydrodynamic forces?) Why or why not? (c) What is the best model of unentangled dynamics to describe the relaxation of this chain? Under this model, what is the friction coefficient for this chain? (i.e., what is R or Z ?) (d) Estimate the longest relaxation time for this chainStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


