Answered step by step
Verified Expert Solution
Question
1 Approved Answer
slov it (d) The following reactions take place in a fixed bed catalyylic reactor. A+BC,r1=r1A,(kmol/kgh)22C2D+2B,r2=r2C,(kmol/kgh) 40%B and 5% inerts. The composition of the product stream
slov it 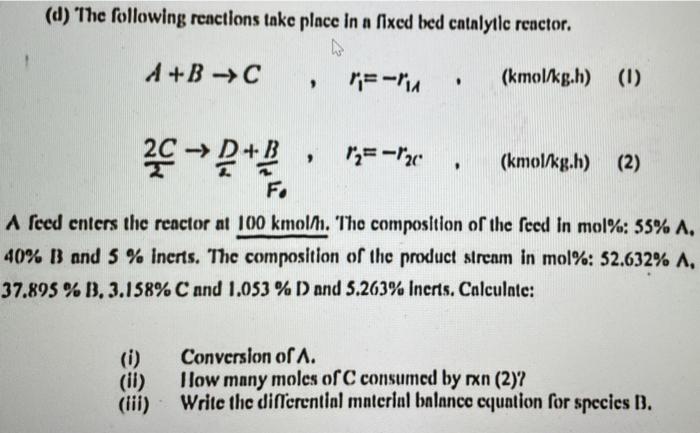
(d) The following reactions take place in a fixed bed catalyylic reactor. A+BC,r1=r1A,(kmol/kgh)22C2D+2B,r2=r2C,(kmol/kgh) 40%B and 5% inerts. The composition of the product stream in mol\%: 52.632%,A. 37.895% B. 3.158\% C and 1.053% D and 5.263% Inerts. Calculate: (i) Conversion of A. (ii) Ilow many moles of C consumed by xxn (2)? (iii) Write the differential materiul balance equation for species B 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


