Answered step by step
Verified Expert Solution
Question
1 Approved Answer
SMC/Physics 23/Fall 2023/Faridian/Exam 3 Part II: Long answers 1. (30 points) On - 1.000 J D Q Th - 500 K C Te -
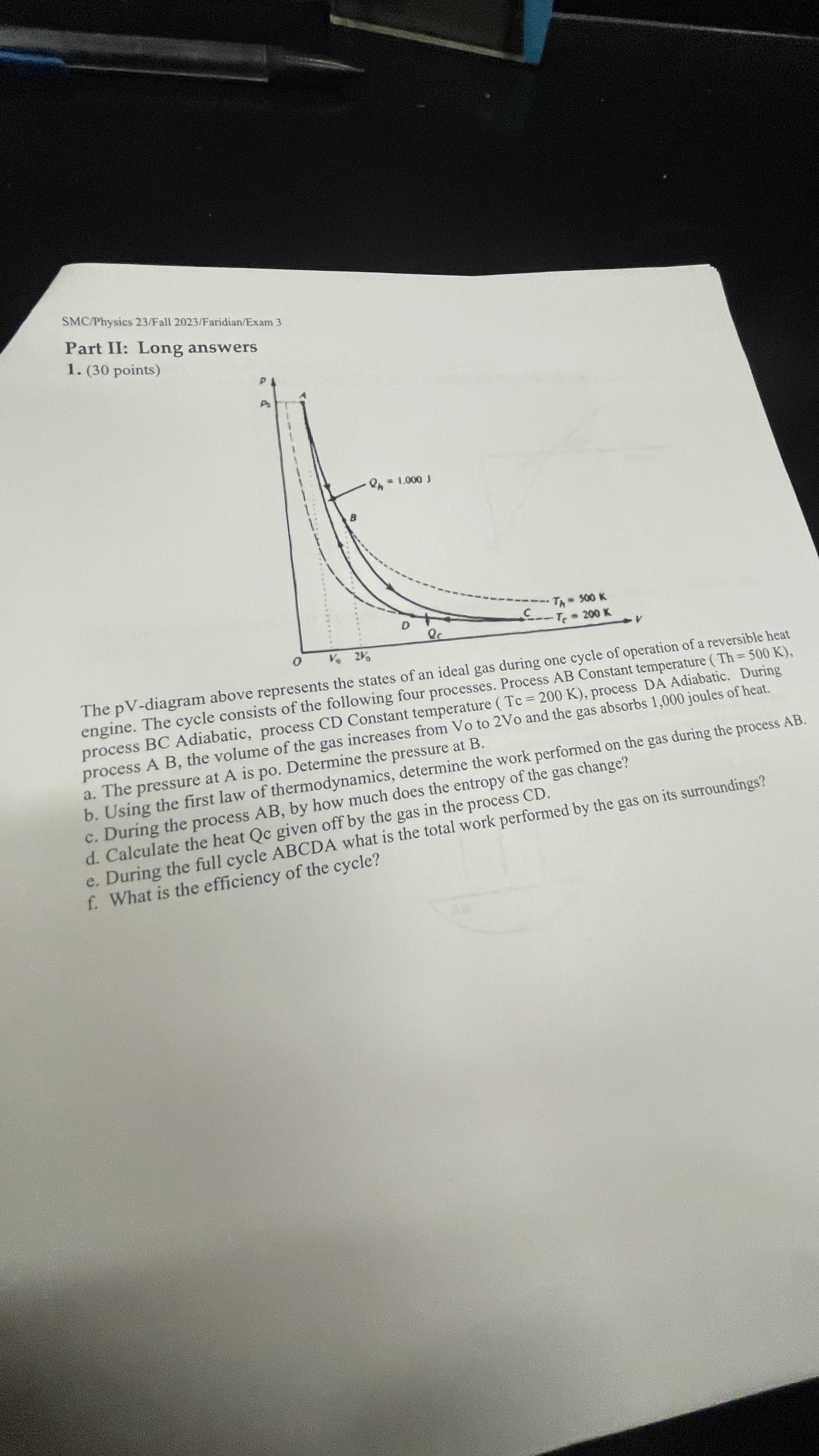
SMC/Physics 23/Fall 2023/Faridian/Exam 3 Part II: Long answers 1. (30 points) On - 1.000 J D Q Th - 500 K C Te - 200 K 0 V. 2% The pV-diagram above represents the states of an ideal gas during one cycle of operation of a reversible heat engine. The cycle consists of the following four processes. Process AB Constant temperature (Th=500 K), process BC Adiabatic, process CD Constant temperature (Tc = 200 K), process DA Adiabatic. During process A B, the volume of the gas increases from Vo to 2Vo and the gas absorbs 1,000 joules of heat. a. The pressure at A is po. Determine the pressure at B. b. Using the first law of thermodynamics, determine the work performed on the gas during the process AB. c. During the process AB, by how much does the entropy of the gas change? d. Calculate the heat Qc given off by the gas in the process CD. e. During the full cycle ABCDA what is the total work performed by the gas on its surroundings? f. What is the efficiency of the cycle? AB
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


