Question
so I did this reaction as follows, sodium dischromate was dissolved in water and sulfuric acid was added to the mixture solution cautiously. this muxture
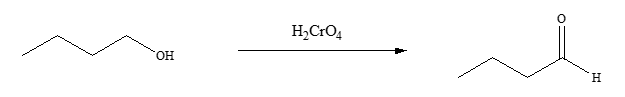
so I did this reaction as follows, sodium dischromate was dissolved in water and sulfuric acid was added to the mixture solution cautiously. this muxture solution was then added to a 250 mL flask containing butanol. this solution was heated and refluxed for 3 hours and then distilled.
i did NMR for the product and what i got was an ester by product and only the size of a Lonnie of Butyraldehyde was produced. i was trying to prove weather or not the distillation is necessary as the reaction occurs as well as if no or less of the acid will be produced.
my question is: i need an explanation of what is causing this ester by product to happen and why? Also how can you fix this problem?
here is 1H and C13 NMR's that i got:
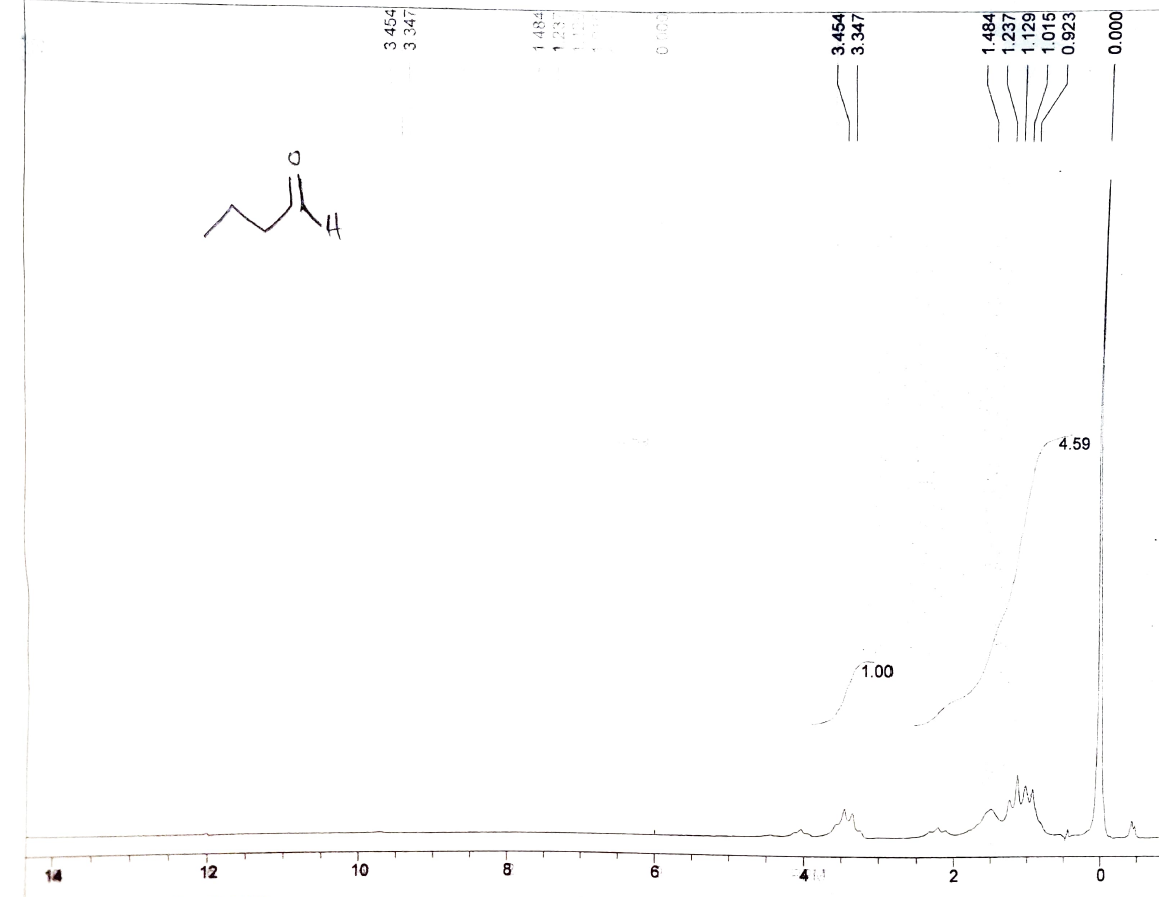
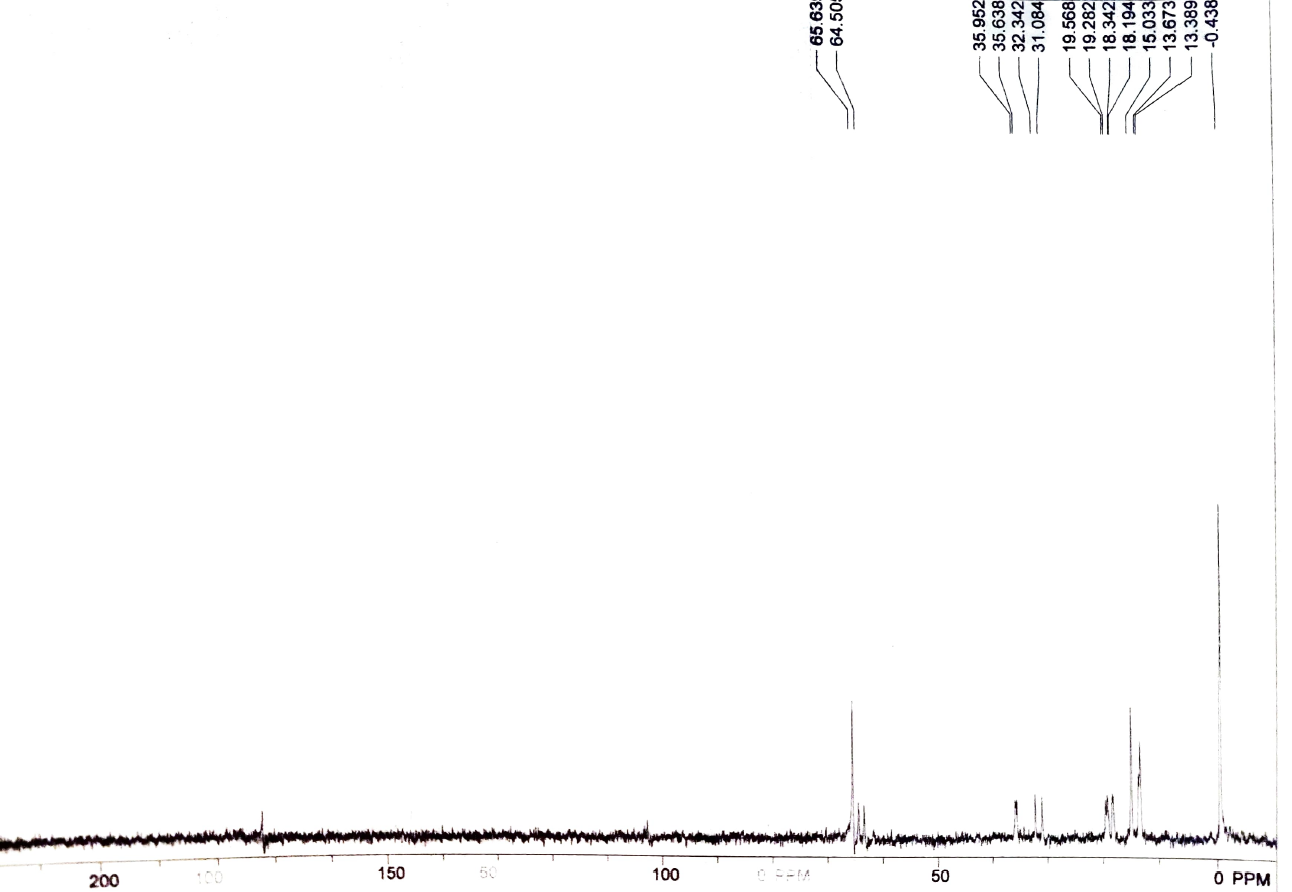
H2CrO4 4.59 14 12 10 8 1.00
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


