Answered step by step
Verified Expert Solution
Question
1 Approved Answer
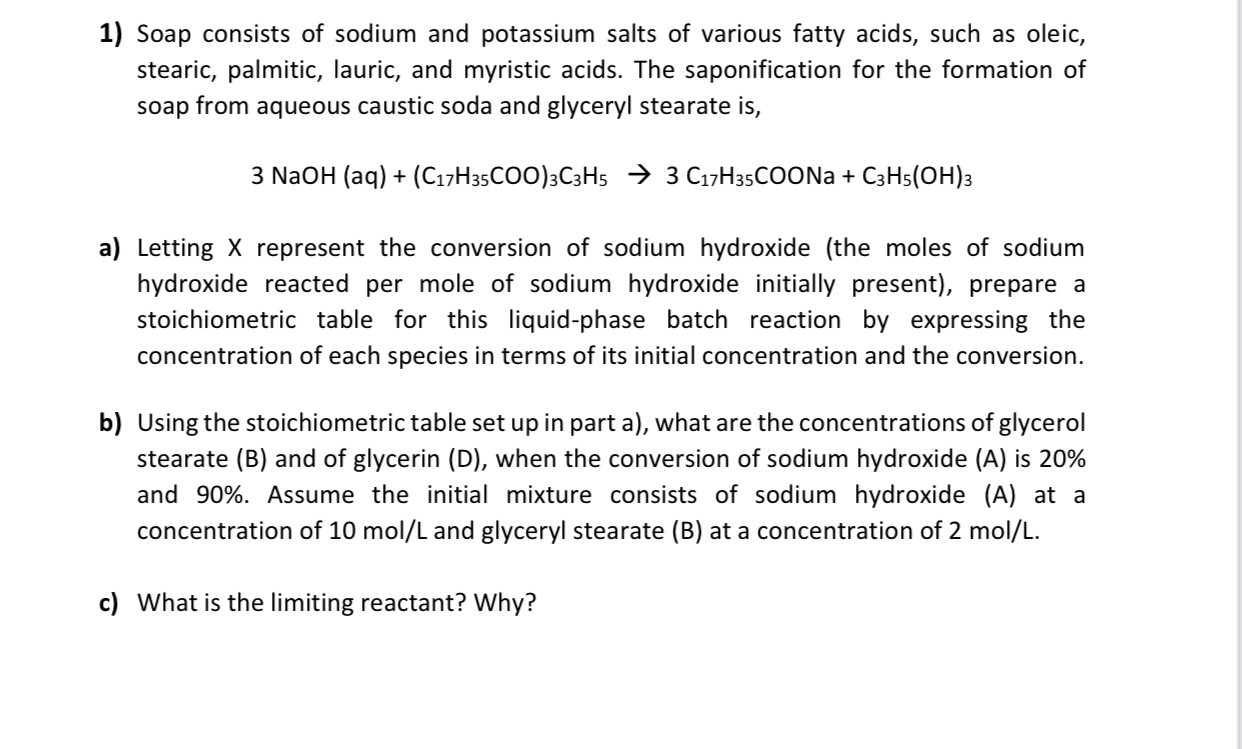
Soap consists of sodium and potassium salts of various fatty acids, such as oleic, stearic, palmitic, lauric, and myristic acids. The saponification for the formation
Soap consists of sodium and potassium salts of various fatty acids, such as oleic, stearic, palmitic, lauric, and myristic acids. The saponification for the formation of soap from aqueous caustic soda and glyceryl stearate is
NaOH
a Letting represent the conversion of sodium hydroxide the moles of sodium hydroxide reacted per mole of sodium hydroxide initially present prepare a stoichiometric table for this liquidphase batch reaction by expressing the concentration of each species in terms of its initial concentration and the conversion.
b Using the stoichiometric table set up in part a what are the concentrations of glycerol stearate B and of glycerin D when the conversion of sodium hydroxide A is and Assume the initial mixture consists of sodium hydroxide A at a concentration of and glyceryl stearate B at a concentration of
c What is the limiting reactant? Why?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


