Sodium sulfate, Na2SO4 with a mass of 15 g is dissolved in 250 g water. What is the boiling point of the solution? Kb
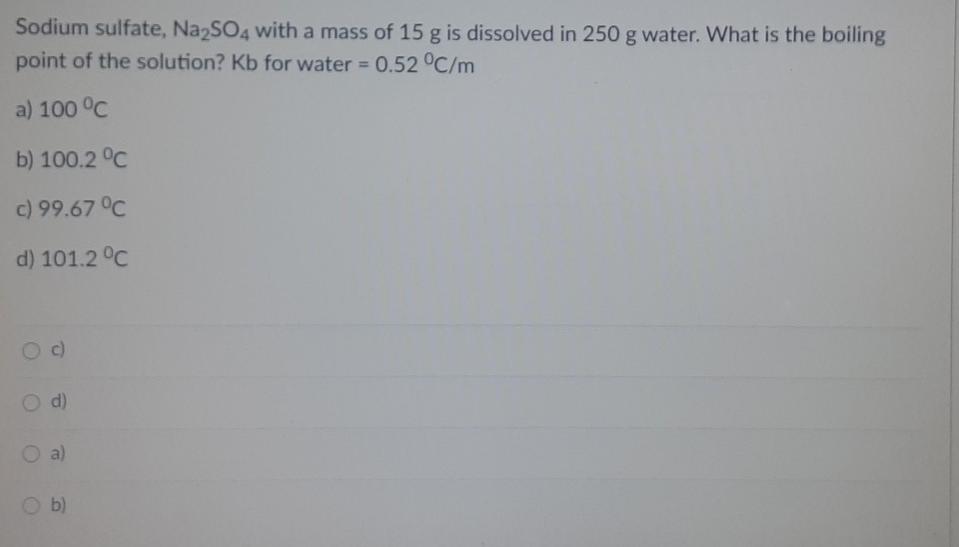


Sodium sulfate, Na2SO4 with a mass of 15 g is dissolved in 250 g water. What is the boiling point of the solution? Kb for water = 0.52 C/m a) 100 C b) 100.2 C c) 99.67 C d) 101.2 C c) d) O a) b) Fifty grams of an unknown nonelectrolyte solute is mixed with 2000 g of naphthalene (C10H8). The boiling point of the solution was observed to be equal to 80C. If the boiling point elevation constant of naphthalene is 6.9 C/m and the boiling point of pure naphthalene is 74.6C: What is the boiling point elevation of the solution? a) 4 C b) 5.0 C c) 5.40 C d) 5.50 C a) b) Od) Fifty grams of an unknown nonelectrolyte solute is mixed with 2000 g of naphthalene (C10H8). The boiling point of the solution was observed to be equal to 80C. If the boiling point elevation constant of naphthalene is 6.9 C/m and the boiling point of pure naphthalene is 74.6C: Compute for the molality of the solution. 0.0783 m O 0.783 m 0.0078 m 0.0782 m
Step by Step Solution
3.40 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
Therefore ...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


