Answered step by step
Verified Expert Solution
Question
1 Approved Answer
SOLUTION AT BOTTOM!!! Can someone explain to me how they got B = 8 0 0 kg / hr in the yellow highlighted portion?? Please
SOLUTION AT BOTTOM!!! Can someone explain to me how they got B kghr in the yellow highlighted portion?? Please for the love of God do NOT copy another solution on here. They're all wrong.
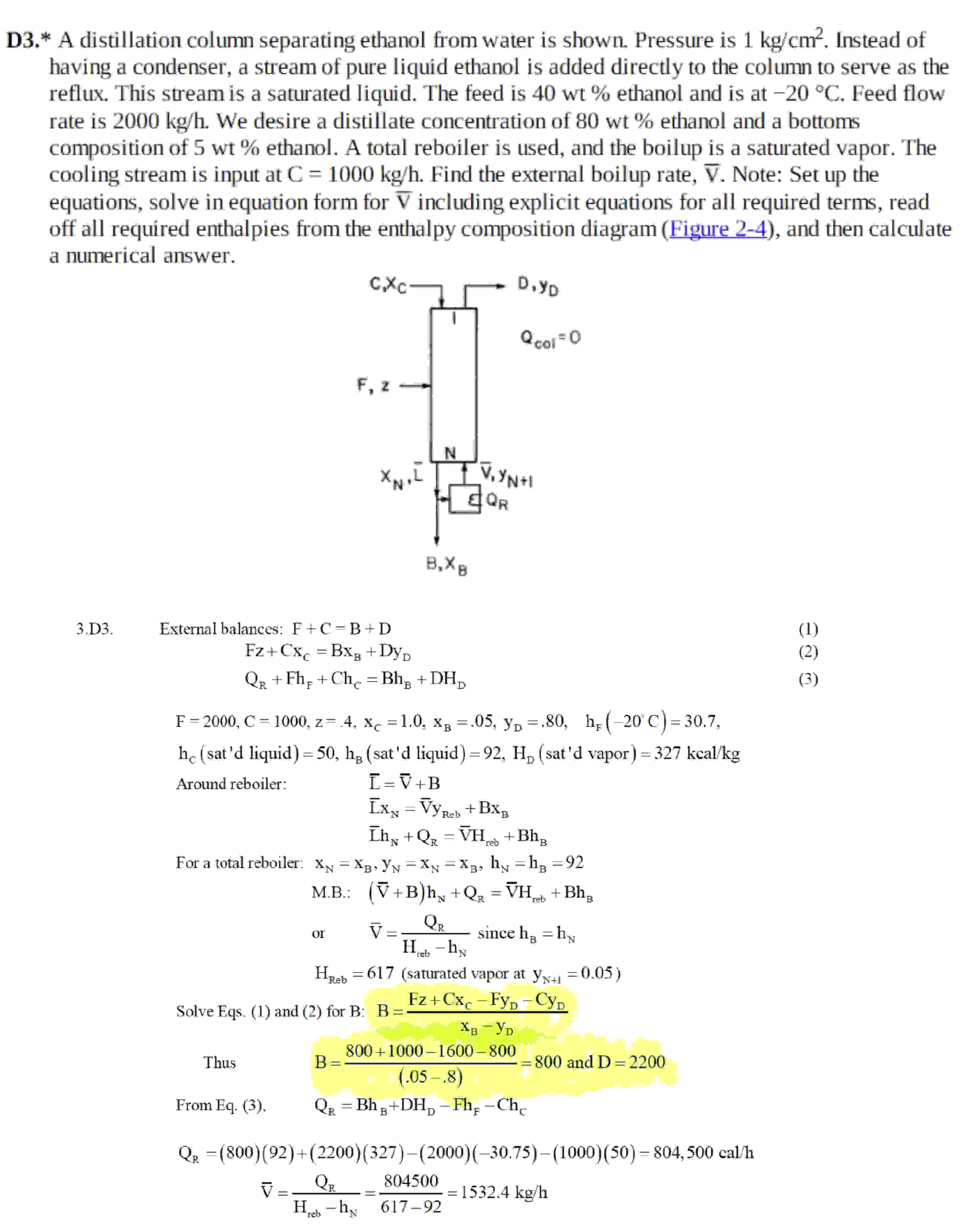
D A distillation column separating ethanol from water is shown. Pressure is Instead of
having a condenser, a stream of pure liquid ethanol is added directly to the column to serve as the
reflux. This stream is a saturated liquid. The feed is ethanol and is at Feed flow
rate is We desire a distillate concentration of ethanol and a bottoms
composition of wt ethanol. A total reboiler is used, and the boilup is a saturated vapor. The
cooling stream is input at Find the external boilup rate, Note: Set up the
equations, solve in equation form for including explicit equations for all required terms, read
off all required enthalpies from the enthalpy composition diagram Figure and then calculate
a numerical answer.
D External balances:
For a total reboiler:
Solve Eqs. and for B:
Thus
and
From Eq
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


