Answered step by step
Verified Expert Solution
Question
1 Approved Answer
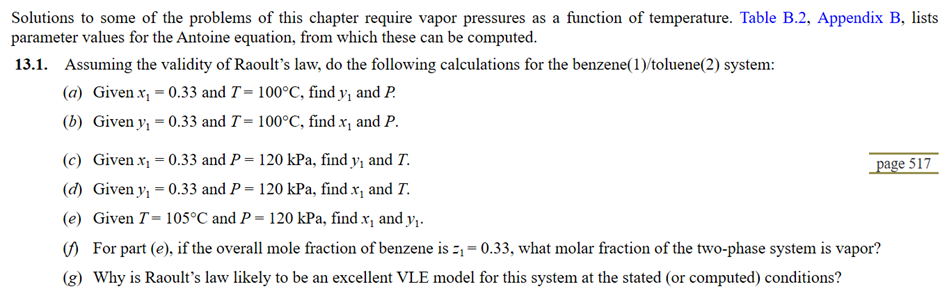
Solutions to some of the problems of this chapter require vapor pressures as a function of temperature. Table B . 2 , Appendix B ,
Solutions to some of the problems of this chapter require vapor pressures as a function of temperature. Table B Appendix B lists
parameter values for the Antoine equation, from which these can be computed.
Assuming the validity of Raoult's law, do the following calculations for the benzenetoluene system:
a Given and find and
b Given and find and
c Given and kPa, find and
d Given and kPa, find and
e Given and kPa, find and
For part e if the overall mole fraction of benzene is what molar fraction of the twophase system is vapor?
g Why is Raoult's law likely to be an excellent VLE model for this system at the stated or computed conditions?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


