Answered step by step
Verified Expert Solution
Question
1 Approved Answer
solve ASAP please give correct answer THE CORRECT ANSWER IS 898.4 not 718.8!! The conversion of aqueous reactant, A, to product, P, proceeds according to
solve ASAP please give correct answer THE CORRECT ANSWER IS 898.4 not 718.8!!
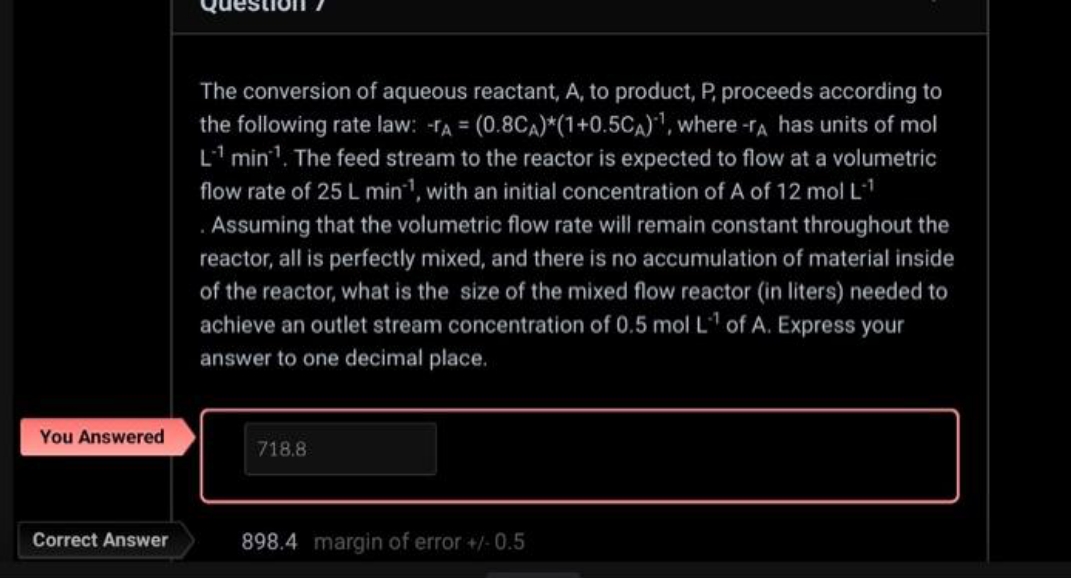
The conversion of aqueous reactant, A, to product, P, proceeds according to the following rate law: TA = (0.8CA)*(1+0.5CA), where-r has units of mol L1 min. The feed stream to the reactor is expected to flow at a volumetric flow rate of 25 L min, with an initial concentration of A of 12 mol L-1 Assuming that the volumetric flow rate will remain constant throughout the reactor, all is perfectly mixed, and there is no accumulation of material inside of the reactor, what is the size of the mixed flow reactor (in liters) needed to achieve an outlet stream concentration of 0.5 mol L of A. Express your answer to one decimal place. You Answered 718.8 Correct Answer 898.4 margin of error +/-0.5
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


