Answered step by step
Verified Expert Solution
Question
1 Approved Answer
solve it A line in the Paschen series occurs at 1089 nm. What is n high for this transition ? A 3 An organic flavoring
solve it 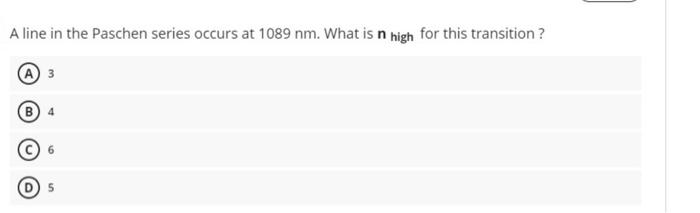
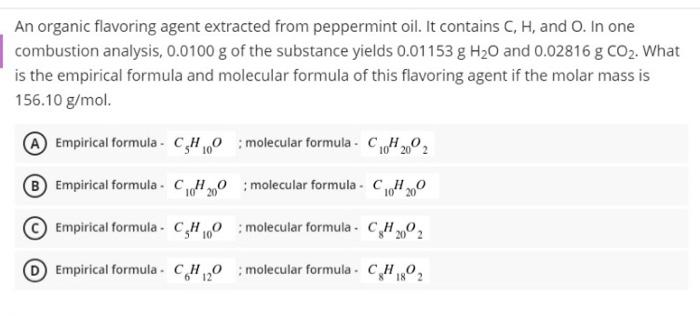

A line in the Paschen series occurs at 1089 nm. What is n high for this transition ? A 3 An organic flavoring agent extracted from peppermint oil. It contains C, H, and O. In one combustion analysis, 0.0100 g of the substance yields 0.01153 g H20 and 0.02816 g CO2. What is the empirical formula and molecular formula of this flavoring agent if the molar mass is 156.10 g/mol. Empirical formula - CHO : molecular formula - CH2002 Empirical formula - CC420 ; molecular formula . CH 10200 Empirical formula. 0,0 molecular formula. C4 2002 Empirical formula - CH20 molecular formula . CH2 6 12 18 The equation for the combustion of glucose (CGH1206) in air is given below: C6H1206 (s) + 602(g) - 6CO2 (g) + 6H20 (1) Calculate the volume of CO2 produced at 37C and 1.00 atm when 4.27 g of glucose is used up in the reaction. A 3.62 B 5.87L 4.16L D 4.48 L 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


