Answered step by step
Verified Expert Solution
Question
1 Approved Answer
SOLVE IT ON A PAPER The following elementary reversible reaction, A+BC+D is carried out in a series of staged packed-bed reactors with inter-stage cooling. The
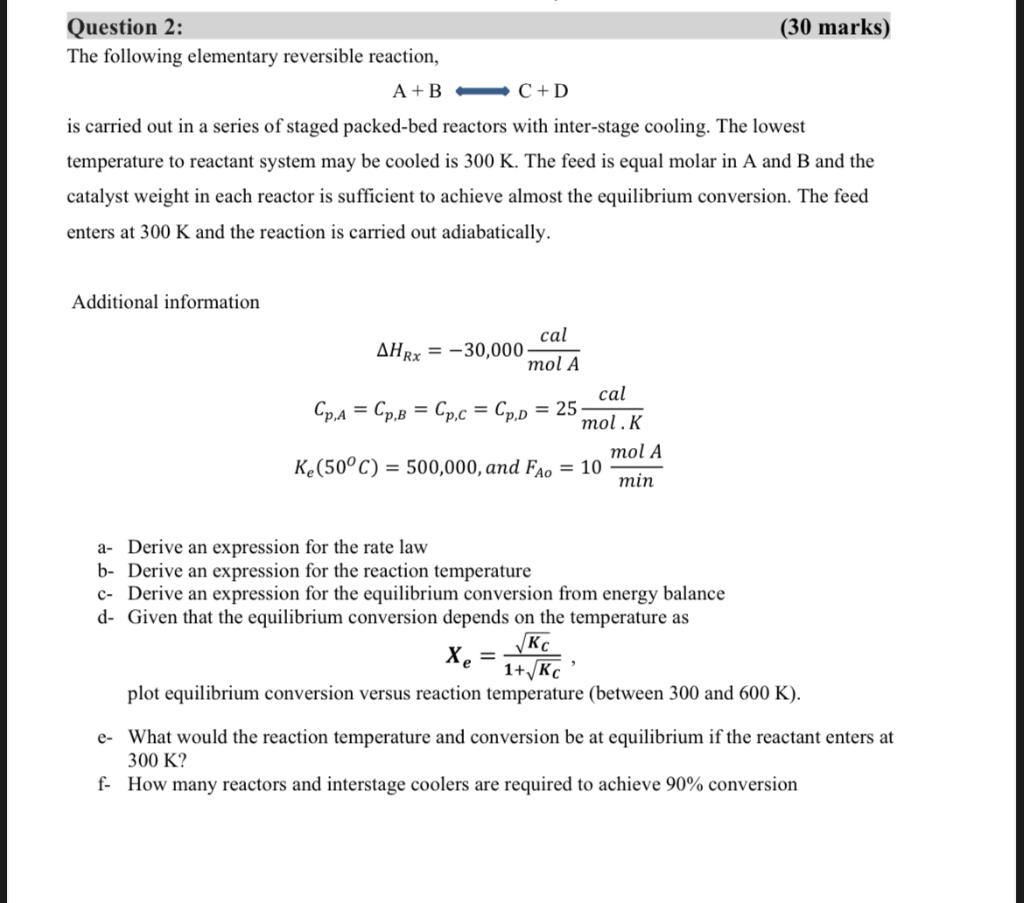
SOLVE IT ON A PAPER
The following elementary reversible reaction, A+BC+D is carried out in a series of staged packed-bed reactors with inter-stage cooling. The lowest temperature to reactant system may be cooled is 300K. The feed is equal molar in A and B and the catalyst weight in each reactor is sufficient to achieve almost the equilibrium conversion. The feed enters at 300K and the reaction is carried out adiabatically. Additional information HRx=30,000molAcalCp,A=Cp,B=Cp,C=Cp,D=25molKcalKe(50C)=500,000,andFAo=10minmolA a- Derive an expression for the rate law b- Derive an expression for the reaction temperature c- Derive an expression for the equilibrium conversion from energy balance d- Given that the equilibrium conversion depends on the temperature as Xe=1+KCKC, plot equilibrium conversion versus reaction temperature (between 300 and 600K ). e- What would the reaction temperature and conversion be at equilibrium if the reactant enters at 300K ? f- How many reactors and interstage coolers are required to achieve 90% conversion The following elementary reversible reaction, A+BC+D is carried out in a series of staged packed-bed reactors with inter-stage cooling. The lowest temperature to reactant system may be cooled is 300K. The feed is equal molar in A and B and the catalyst weight in each reactor is sufficient to achieve almost the equilibrium conversion. The feed enters at 300K and the reaction is carried out adiabatically. Additional information HRx=30,000molAcalCp,A=Cp,B=Cp,C=Cp,D=25molKcalKe(50C)=500,000,andFAo=10minmolA a- Derive an expression for the rate law b- Derive an expression for the reaction temperature c- Derive an expression for the equilibrium conversion from energy balance d- Given that the equilibrium conversion depends on the temperature as Xe=1+KCKC, plot equilibrium conversion versus reaction temperature (between 300 and 600K ). e- What would the reaction temperature and conversion be at equilibrium if the reactant enters at 300K ? f- How many reactors and interstage coolers are required to achieve 90% conversionStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


