Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Solve only b please!!!!!!!!!!!!!!!!!!!!! The irreversible gas-phase reaction AcatalystB is carried out over a packed bed of solid catalyst particles. The reaction is first order
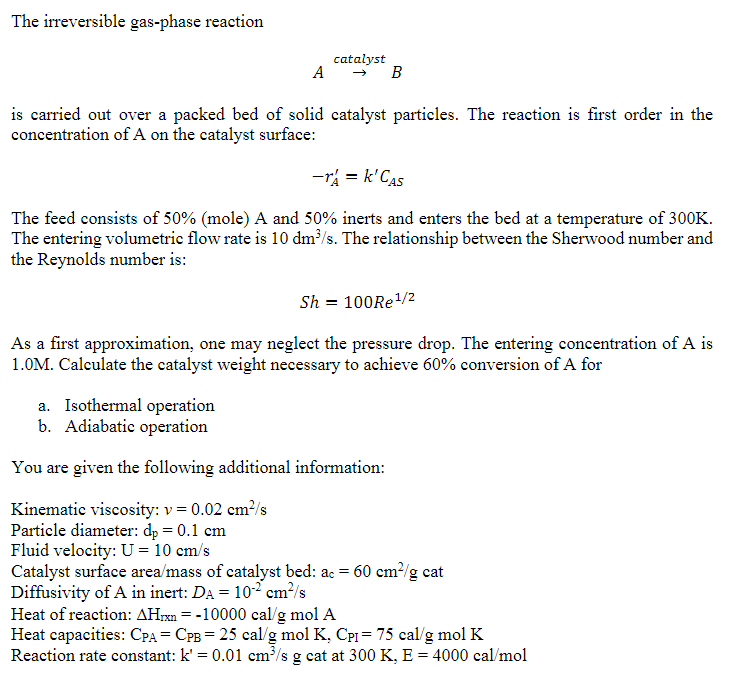
Solve only b please!!!!!!!!!!!!!!!!!!!!!
The irreversible gas-phase reaction AcatalystB is carried out over a packed bed of solid catalyst particles. The reaction is first order in the concentration of A on the catalyst surface: rA=kCAS The feed consists of 50% (mole) A and 50% inerts and enters the bed at a temperature of 300K. The entering volumetric flow rate is 10dm3/s. The relationship between the Sherwood number and the Reynolds number is: Sh=100Re1/2 As a first approximation, one may neglect the pressure drop. The entering concentration of A is 1.0M. Calculate the catalyst weight necessary to achieve 60% conversion of A for a. Isothermal operation b. Adiabatic operation You are given the following additional information: Kinematic viscosity: v=0.02cm2/s Particle diameter: dp=0.1cm Fluid velocity: U=10cm/s Catalyst surface area / mass of catalyst bed: ac=60cm2/g cat Diffusivity of A in inert: DA=102cm2/s Heat of reaction: Hrxn=10000cal/gmolA Heat capacities: CPA=CPB=25cal/gmolK,CPI=75cal/gmolK Reaction rate constant: k=0.01cm3/sg cat at 300K,E=4000cal/molStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


