Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Solve part c and d please. Thank you The table on the following page shows equilibrium distribution data for several gaseous solutes dissolved in water,

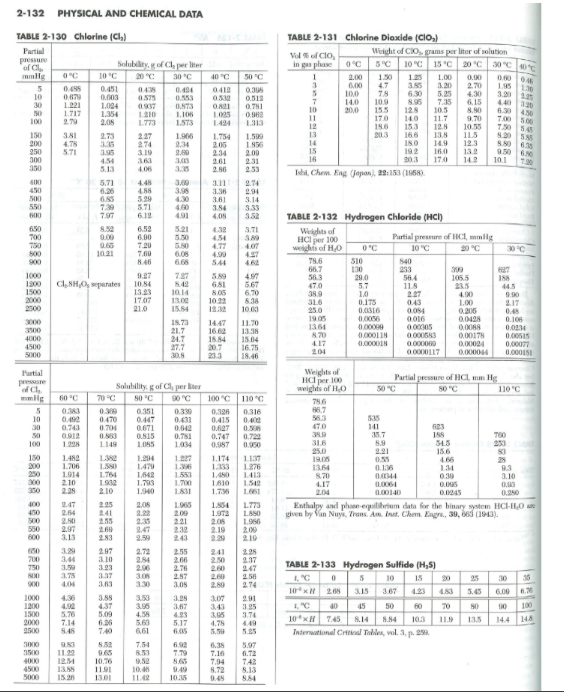
Solve part c and d please.
Thank you
The table on the following page shows equilibrium distribution data for several gaseous solutes dissolved in water, with air as the carrier gas, taken from Perry's Chemical Engineer's Handbook, 8th Ed. For this question, please include your plot(s) with your complete homework submission in one file for full credit! a. For a chlorine liquid stripping process, graph the equilibrium distribution data at 293K, 323K, and 373K, from a partial pressure in the gas phase of 0mmHg to 400mmHg. The total system pressure is 1atm. On the plot, show the molar concentration of chlorine dissolved in water ( mol/L) vs. the partial pressure of chlorine in the gas phase (atm). How does the solubility of chlorine in water change with an increase in temperature? (2 pts) b. At current conditions for liquid stripping at 323K, the partial pressure of chlorine (A) in the bulk gas phase is pA=0.1atm, and the concentration of chlorine in the bulk liquid phase is cAL=0.03mol/L. The liquid film mass-transfer coefficient for Cl2 in water is kL=1.0m/s and the gas film mass-transfer coefficient for Cl2 in air is kG=0.1kgmole/m2satm at 323K. Add to your plot the operating point and the operating line kL/kG for this system. Estimate the compositions at the gas/liquid interface (pA,i and cAL,i) using your equilibrium plot. (2 pts) c. Calculate the overall mass-transfer coefficient, KL. Is this process controlled by gas-phase or liquid-phase convection, or neither? ( 2 pts) d. What is the steady-state molar flux of chlorine, NA, for this process? ( 2 pts) 2-132 PHYSICAL AND CHEMICAL DATA TABLE 2-130 Chlorine (Cl2) TAbLe 2-131 Chlorine Dioxide (ClO2) TABLE 2-132 Hydrogen Chloride (HCl) Enthalyy and phese-equilurium dafa for the binary system HCl2H4O ase given by lan Nuys, Thans. Are Inst. Chem. Engro, 30, 660 (1913). TABLE 2-133 Hydrogen Sulfide (H25) Intenectionel Critiol Takes, wa. 3, p. 25Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


