Question
Solve the following experiment. Website for carrying out the experiment( https://www.geogebra.org/m/P8Gj2RNF ) 1. Determine the metal under test Aluminium; 2. Choose a mass value for
Solve the following experiment. Website for carrying out the experiment( https://www.geogebra.org/m/P8Gj2RNF )
1. Determine the metal under test "Aluminium";
2. Choose a mass value for the metal;
3. Turn the Bunsen Burner "OFF ON"4. Note the temperature of the Becker when stabilizer (Metal temperature);
5. Determine the temperature of the water inside the calorimeter (4C to 20C);
6. Determine a mass of water inside the calorimeter;
7. Drag the selector from "Becker" to "calorimeter";
8. Note that the temperature inside the calorimeter will rise until it stabilizes. annotate astabilization temperature ??.
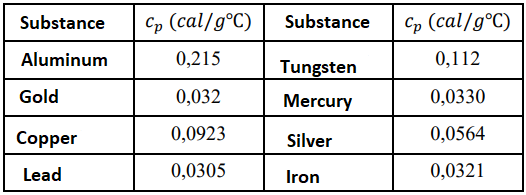
9. Determine the specific heat ? of the solid and compare with the tabulated value (see Table1). Comment your result.
10. Redo for Copper and Gold metals.
11. Remember that the specific heat of water is 1 ???/??
Table 1 - Specific heats of some solids at constant pressure of ? ??? and room temperature.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


